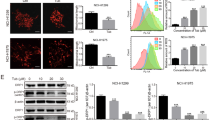
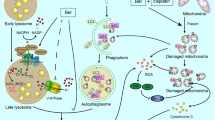
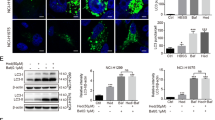
Abstract
Numerous approaches suggested that compounds with conjugated triazole moieties or benzoxazone pharmacores are effective to antagonize proliferation of human tumors. The current study reported that a synthetic triazole-conjugated benzoxazone, 4-((5-benzyl-1H-1,2,3-triazol-3-yl)-methyl)-7-methoxy-2H-benzo[b][1,4]-oxazin-3(4H)-one (BTO), inhibited growth rates of human non-small cell lung cancer cells. The cytotoxicity can be enhanced with increasing drug concentrations. More evidence supported that the induced reactive oxygen species lead to ultimate apoptotic cell death by recruiting autophagy. The mechanistic pathway as elucidated involved tumor suppressor p53 activation and LC3-1 conversion followed by PARP and procaspase-3 cleavage. Autophagy inhibition reverted apoptotic death and restored cell viabilities. BTO suppressed the development of A549 cell xenograft tumors by activating autophagy and apoptosis simultaneously. As an efficient tumor growth inhibitor with relatively small molecular weight, BTO is a viable addition to the existing list of lung cancer treatment.








Similar content being viewed by others
Abbreviations
- Bax:
-
B-cell lymphoma-2-associated X-protein
- BTO:
-
4-((5-Benzyl-1H-1,2,3-triazol-3-yl)-methyl)-7-methoxy-2H-benzo[b][1,4]-oxazin-3(4H)-one
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- DCFH-DA:
-
2′,7′-Dichlorofluorescein diacetate
- DMEM:
-
Dulbecco’s modified eagle medium
- DMSO:
-
Dimethyl sulfoxide
- EDTA:
-
Ethylenediaminetetraacetic acid
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GFP:
-
Green fluorescence protein
- HE:
-
Hematoxylin and eosin
- HRP:
-
Horseradish peroxidase
- LC3:
-
Microtubule-associated protein light chain 3
- 3-MA:
-
3-Methyladenine
- MTT:
-
3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyltetrazoliumbromide
- NAC:
-
N-Acetylcysteine
- NSCLC:
-
Non-small-cell lung carcinomas
- PARP:
-
Poly(adenosine diphosphate ribose) polymerase
- PBS:
-
Phosphate-buffered saline
- PCNA:
-
Proliferating cell nuclear antigen
- PI:
-
Propidium iodide
- PI3K:
-
Phosphoinositide 3-kinase
- ROS:
-
Reactive oxygen species
- SD:
-
Standard deviation
- TRITC:
-
Tetramethylrhodamine isocyanate
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386. https://doi.org/10.1002/ijc.29210
Neal RD, Hamilton W, Rogers TK (2014) Lung cancer. BMJ 349. https://doi.org/10.1136/bmj.g6560
de Bruin EC, Medema JP (2008) Apoptosis and non-apoptotic deaths in cancer development and treatment response. Cancer Treat Rev 34(8):737–749. http://doi.org/10.1016/j.ctrv.2008.07.001
Reddy DM, Srinivas J, Chashoo G, Saxena AK, Sampath Kumar HM (2011) 4β-[(4-alkyl)-1,2,3-triazol-1-yl] podophyllotoxins as anticancer compounds: design, synthesis and biological evaluation. Eur J Med Chem 46(6):1983–1991. https://doi.org/10.1016/j.ejmech.2011.02.016
Zhao L, Mao L, Hong G, Yang X, Liu T (2015) Design, synthesis and anticancer activity of matrine–1H-1,2,3-triazole–chalcone conjugates. Bioorg Med Chem Lett 25(12):2540–2544. https://doi.org/10.1016/j.bmcl.2015.04.051
Odlo K, Hentzen J, dit Chabert JF, Ducki S, Gani OABSM., Sylte I, Skrede M, Flørenes VA, Hansen TV (2008) 1,5-Disubstituted 1,2,3-triazoles as cis-restricted analogues of combretastatin A-4: synthesis, molecular modeling and evaluation as cytotoxic agents and inhibitors of tubulin. Bioorg Med Chem Lett 16(9):4829–4838. https://doi.org/10.1016/j.bmc.2008.03.049
Borate HB, Maujan SR, Sawargave SP, Chandavarkar MA, Vaiude SR, Joshi VA, Wakharkar RD, Iyer R, Kelkar RG, Chavan SP, Kunte SS (2010) Fluconazole analogues containing 2H-1,4-benzothiazin-3(4H)-one or 2H-1,4-benzoxazin-3(4H)-one moieties, a novel class of anti-candida agents. Bioorg Med Chem Lett 20(2):722–725. https://doi.org/10.1016/j.bmcl.2009.11.071
Fringuelli R, Giacchè N, Milanese L, Cenci E, Macchiarulo A, Vecchiarelli A, Costantino G, Schiaffella F (2009) Bulky 1,4-benzoxazine derivatives with antifungal activity. Bioorg Med Chem Lett 17(11):3838–3846. https://doi.org/10.1016/j.bmc.2009.04.051
Su CL, Tseng CL, Ramesh C, Liu HS, Huang CF, Yao CF (2017) Using gene expression database to uncover biology functions of 1,4-disubstituted 1,2,3-triazole analogues synthesized via a copper(I)-catalyzed reaction. Eur J Med Chem 132:90–107. https://doi.org/10.1016/j.ejmech.2017.03.034
Liu CY, Wu PT, Wang JP, Fan PW, Hsieh CH, Su CL, Chiu CC, Yao CF, Fang K (2015) An indolylquinoline derivative promotes apoptosis in human lung cancer cells by impairing mitochondrial functions. Apoptosis 20(11):1471–1482. https://doi.org/10.1007/s10495-015-1165-6
Ding Y, Nguyen TA (2013) PQ1, a quinoline derivative, induces apoptosis in T47D breast cancer cells through activation of caspase-8 and caspase-9. Apoptosis 18(9):1071–1082. https://doi.org/10.1007/s10495-013-0855-1
Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Saavedra E, Moreno-Sanchez R (2010) The causes of cancer revisited: “mitochondrial malignancy” and ROS-induced oncogenic transformation: why mitochondria are targets for cancer therapy. Mol Asp Med 31(2):145–170. https://doi.org/10.1016/j.mam.2010.02.008
Liu G, Pei F, Yang F, Li L, Amin AD, Liu S, Buchan JR, Cho WC (2017) Role of autophagy and apoptosis in non-small-cell lung cancer. Int J Mol Sci 18(2):367. https://doi.org/10.3390/ijms18020367
Hong S-O, Choi IK, Jeong W, Lee SR, Sung HJ, Hong SS, Seo JH (2017) Ulmus davidiana Nakai induces apoptosis and autophagy on non-small cell lung cancer cells. J Ethnopharmacol 202:1–11. https://doi.org/10.1016/j.jep.2017.03.009
Burris HA 3rd (2009) Shortcomings of current therapies for non-small-cell lung cancer: unmet medical needs. Oncogene 1(28 Suppl):S4–13. https://doi.org/10.1038/onc.2009.196
Chang A (2011) Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer 71(1):3–10. https://doi.org/10.1016/j.lungcan.2010.08.022
Bollu R, Palem JD, Bantu R, Guguloth V, Nagarapu L, Polepalli S, Jain N (2015) Rational design, synthesis and anti-proliferative evaluation of novel 1,4-benzoxazine-[1,2,3]triazole hybrids. Eur J Med Chem 89:138–146. https://doi.org/10.1016/j.ejmech.2014.10.051
Choi AM, Ryter SW, Levine B (2013) Autophagy in human health and disease. N Engl J Med 368(19):1845–1846. https://doi.org/10.1056/NEJMc1303158
Dielschneider RF, Henson ES, Gibson SB (2017) Lysosomes as oxidative targets for cancer therapy. Oxid Med Cell Longev 2017:3749157. https://doi.org/10.1155/2017/3749157
Hasima N, Ozpolat B (2014) Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis 5(11):e1509. https://doi.org/10.1038/cddis.2014.467
Liu G, Pei F, Yang F, Li L, Amin AD, Liu S, Buchan JR, Cho WC (2017) Role of autophagy and apoptosis in non-small-cell lung cancer. Int J Mol Sci 18(2):367. https://doi.org/10.3390/ijms18020367
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen E-L, Mizushima N, Ohsumi Y, Cattoretti G, Levine B (2003) Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 112(12):1809–1820. https://doi.org/10.1172/JCI20039
Strohecker AM, White E (2014) Autophagy promotes BrafV600E-driven lung tumorigenesis by preserving mitochondrial metabolism. Autophagy 10(2):384–385. https://doi.org/10.4161/auto.27320
White E (2012) Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 12(6):401–410
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8 (9):741–752. http://www.nature.com/nrm/journal/v8/n9/suppinfo/nrm2239_S1.html
Liu F, Liu D, Yang Y, Zhao S (2013) Effect of autophagy inhibition on chemotherapy-induced apoptosis in A549 lung cancer cells. Oncol Lett 5(4):1261–1265. https://doi.org/10.3892/ol.2013.1154
Tanida I, Ueno T, Kominami E (2004) LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36(12):2503–2518. https://doi.org/10.1016/j.biocel.2004.05.009
Luo S, Rubinsztein DC (2010) Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ 17(2):268–277. https://doi.org/10.1038/cdd.2009.121
Schumacker PT Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10 (3):175–176. https://doi.org/10.1016/j.ccr.2006.08.015
Kaminskyy VO, Zhivotovsky B (2014) Free radicals in cross talk between autophagy and apoptosis. Antioxid Redox Signal 21(1):86–102. https://doi.org/10.1089/ars.2013.5746
Chang C-T, Hseu Y-C, Thiyagarajan V, Lin K-Y, Way T-D, Korivi M, Liao J-W, Yang H-L (2017) Chalcone flavokawain B induces autophagic-cell death via reactive oxygen species-mediated signaling pathways in human gastric carcinoma and suppresses tumor growth in nude mice. Arch Toxicol. 91(10):3341–3364. https://doi.org/10.1007/s00204-017-1967-0
Jin CY, Yu HY, Park C, Han MH, Hong SH, Kim KS, Lee YC, Chang YC, Cheong J, Moon SK, Kim GY, Moon HI, Kim WJ, Lee JH, Choi YH (2013) Oleifolioside B-mediated autophagy promotes apoptosis in A549 human non-small cell lung cancer cells. Int J Oncol 43(6):1943–1950. https://doi.org/10.3892/ijo.2013.2143
Wang K (2015) Autophagy and apoptosis in liver injury. Cell Cycle 14(11):1631–1642. https://doi.org/10.1080/15384101.2015.1038685
Ma G, Luo W, Lu J, Ma DL, Leung CH, Wang Y, Chen X (2016) Cucurbitacin E induces caspase-dependent apoptosis and protective autophagy mediated by ROS in lung cancer cells. Chem Biol Interact 253:1–9. https://doi.org/10.1016/j.cbi.2016.04.028
Kulkarni YM, Kaushik V, Azad N, Wright C, Rojanasakul Y, O’Doherty G, Iyer AK (2016) Autophagy-Induced apoptosis in lung cancer cells by a novel digitoxin analog. J Cell Physiol 231(4):817–828. https://doi.org/10.1002/jcp.25129
Wirawan E, Vande Walle L, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, Roelandt R, De Rycke R, Verspurten J, Declercq W, Agostinis P, Vanden Berghe T, Lippens S, Vandenabeele P (2010) Caspase-mediated cleavage of beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis 1(1):e18. https://doi.org/10.1038/cddis.2009.16
Baburajeev CP, Mohan CD, Rangappa S, Mason DJ, Fuchs JE, Bender A, Barash U, Vlodavsky I, Basappa, Rangappa KS (2017) Identification of novel class of triazolo-thiadiazoles as potent inhibitors of human heparanase and their anticancer activity. BMC Cancer 17(1):235. https://doi:10.1186/s12885-017-3214-8
Acknowledgements
The construct GFP-LC3 was kindly provided by professor Huang Wei-Bang, Department of Life Science, National Taiwan University, Taipei, Taiwan. The work is supported by grants from National Taiwan Normal University (106T3040B2, 106T3040C2 and 106T3040D2). Technical assistance of College of Life Science and Instrumentation Center, National Taiwan Normal University with the confocal laser microscopy is appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
About this article
Cite this article
Hsieh, CH., Wang, JP., Chiu, CC. et al. A triazole-conjugated benzoxazone induces reactive oxygen species and promotes autophagic apoptosis in human lung cancer cells. Apoptosis 23, 1–15 (2018). https://doi.org/10.1007/s10495-017-1432-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1432-9