Abstract
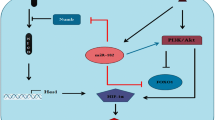
Trastuzumab (Herceptin) monoclonal antibody directed against HER2 receptor has been administered as a treatment for metastatic HER2 positive breast cancer. The problematic issue in treatment of HER2 positive breast cancer cells is commonly the induction of resistance to trastuzumab which might be due to modulation of some vital signaling elements such as Notch1 and Pin1. In this study, we were aimed to investigate whether the cross talk between pin1 and Notch1 has a role in this event. Our results indicated that the expression level of Pin1 in resistant SKBR3 cells increased by about twofold relative to sensitive SKBR3 cells. Besides, Pin1 inhibition via juglone reduced the extent of proliferation, colony formation and migration capacity of resistant SKBR3 cells. In addition, despite a feed forward loop between Notch1 and Pin1 in sensitive SKBR3 cells, inhibition of Notch1 cleavage in resistant SKBR3 cells did not affect pin1 level whereas pin1 inhibition by juglone reduced the level of Hes1, p-Akt and increased the cellular content of Numb. Therefore, we concluded that pin1 inhibition could be considered as a promising sensitizing strategy to weaken trastuzumab resistance.







Similar content being viewed by others
References
Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2(2):127–137
Graus-Porta D et al (1997) ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J 16(7):1647–1655
Olayioye MA et al (2000) The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19(13):3159–3167
Hynes NE, Stern DF (1994) The biology of erbB-2/nue/HER-2 and its role in cancer. Biochim Biophys Acta (BBA) Rev Cancer 1198(2):165–184
Harari D, Yarden Y (2000) Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene 19(53):6102–6114
Musgrove EA et al (1994) Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci 91(17):8022–8026
Slamon DJ et al (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235(4785):177–182
Ross JS, Fletcher JA (1998) The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem cells 16(6):413–428
Vogel CL et al (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20(3):719–726
Slamon D et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N EnglJ Med 344:783–792
Slonim DK (1999) Transcriptional profiling in cancer: the path to clinical pharmacogenomics. Pharmacol Ther 82(2–3):241–250
Nahta R, Esteva FJ (2006) Herceptin: mechanisms of action and resistance. Cancer Lett 232(2):123–138
Cohen B et al (2010) Cyclin D1 is a direct target of JAG1-mediated Notch signaling in breast cancer. Breast Cancer Res Treat 123(1):113–124
Takahashi K et al (2008) Prolyl isomerase, Pin1: new findings of post-translational modifications and physiological substrates in cancer, asthma and Alzheimer’s disease. CMLS Cell Mol Life Sci 65(3):359–375
Ryo A et al (2003) Prolyl isomerase Pin1: a catalyst for oncogenesis and a potential therapeutic target in cancer. J Cell Sci 116(5):773–783
Monje P et al (2005) Regulation of the transcriptional activity of c-Fos by ERK A novel role for the prolyl isomerase PIN1. J Biol Chem 280(42):35081–35084
Lee NY et al (2009) The prolyl isomerase Pin1 interacts with a ribosomal protein S6 kinase to enhance insulin-induced AP-1 activity and cellular transformation. Carcinogenesis 30(4):671–681
Lu KP, Zhou XZ (2007) The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol 8(11):904–916.
Rustighi A et al (2009) The prolyl-isomerase Pin1 is a Notch1 target that enhances Notch1 activation in cancer. Nat Cell Biol 11(2):133–142
Wulf G et al (2003) The prolyl isomerase Pin1 in breast development and cancer. Breast Cancer Res 5(2):76–88
Lam PB et al (2008) Prolyl isomerase Pin1 is highly expressed in Her2-positive breast cancer and regulates erbB2 protein stability. Mol Cancer 7(1):91
Aithal KB et al (2012) Tumor growth inhibitory effect of juglone and its radiation sensitizing potential in vivo and in vitro studies. Integr Cancer Ther 11(1):68–80
Sugie S et al (1998) Inhibitory effects of plumbagin and juglone on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett 127(1):177–183
Vistica DT et al (1991) Tetrazolium-based assays for cellular viability: a critical examination of selected parameters affecting formazan production. Cancer Res 51(10):2515–2520
Chou T-C, Talalay P (1984) Quantitative analysis of dose–effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Meshkini A, Yazdanparast R (2008) Involvement of ERK/MAPK pathway in megakaryocytic differentiation of K562 cells induced by 3-hydrogenkwadaphnin. Toxicol In Vitro 22(6):1503–1510
Liang K et al (2003) Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther 2(11):1113–1120
Lowry OH et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275.
Nahta R et al (2005) Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 65(23):11118–11128
Osipo C et al (2008) ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a γ-secretase inhibitor. Oncogene 27(37):5019–5032
Sajadimajd S, Yazdanparast R (2015) Differential behaviors of trastuzumab-sensitive and-resistant SKBR3 cells treated with menadione reveal the involvement of Notch1/Akt/FOXO1 signaling elements. Mol Cell Biochem 408(1–2):89–102
Liao Y et al (2009) Peptidyl-prolyl cis/trans isomerase Pin1 is critical for the regulation of PKB/Akt stability and activation phosphorylation. Oncogene 28(26):2436–2445
Atkinson G et al (2009) The prolyl isomerase Pin1 regulates the NF-κB signaling pathway and interleukin-8 expression in glioblastoma. Oncogene 28(42):3735–3745
Cheng C-W et al (2013) PIN1 inhibits apoptosis in hepatocellular carcinoma through modulation of the antiapoptotic function of survivin. Am J Pathol 182(3):765–775
Chakrabarty A et al (2013) Trastuzumab-resistant cells rely on a HER2-PI3K-FoxO-survivin axis and are sensitive to PI3K inhibitors. Cancer Res 73(3):1190–1200
Storz P (2011) Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid Redox Signal 14(4):593–605
Acknowledgments
The authors appreciate the joint financial support of this investigation by the Research Council of University of Tehran, Iran National Science Foundation (INSF) and National Institute of Genetic Engineering and Biotechnology (NIGEB).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sajadimajd, S., Yazdanparast, R. Sensitizing effect of juglone is mediated by down regulation of Notch1 signaling pathway in trastuzumab-resistant SKBR3 cells. Apoptosis 22, 135–144 (2017). https://doi.org/10.1007/s10495-016-1291-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-016-1291-9