Abstract
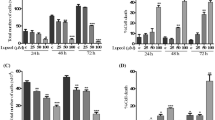
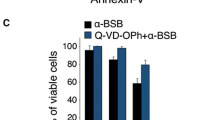
The antiproliferative and cytotoxic activity of glucolaxogenin and its ability to induce apoptosis and autophagy in cervical cancer cells are reported. We ascertained that glucolaxogenin exerts an inhibitory effect on the proliferation of HeLa, CaSki and ViBo cells in a dose-dependent manner. Analysis of DNA distribution in the cell-cycle phase of tumor cells treated with glucolaxogenin suggests that the anti-proliferative activity of this steroid is not always dependent on the cell cycle. Cytotoxic activity was evaluated by detection of the lactate dehydrogenase enzyme in supernatants from tumor cell cultures treated with the steroid. Glucolaxogenin exhibited null cytotoxic activity. With respect to the apoptotic activity, the generation of apoptotic bodies, the presence of active caspase-3 and annexin-V, as well as the DNA fragmentation observed in all tumor lines after treatment with glucolaxogenin suggests that this compound does indeed induce cell death by apoptosis. Also, a significantly increased presence of the LC3-II, LC3 and Lamp-1 proteins was evidenced with the ultrastructural existence of autophagic vacuoles in cells treated with this steroidal glycoside, indicating that glucolaxogenin also induces autophagic cell death. It is important to note that this compound showed no cytotoxic effect and did not affect the proliferative capacity of mononuclear cells obtained from normal human peripheral blood activated by phytohaemagglutinin. Thus, glucolaxogenin is a compound with anti-proliferative properties that induces programmed cell death in cancer cell lines, though it is selective with respect to normal lymphocytic cells. These findings indicate that this glycoside could have a selective action on tumor cells and, therefore, be worthy of consideration as a therapeutic candidate with anti-tumor potential.













Similar content being viewed by others
References
Okanishi T, Akahori A, Yasuda F (1965) Studies on the steroidal components of domestic plants. XLVII. Constituents of the stem of Smilax sieboldi Miq. (1). The structure of laxogenin. Chem Pharm Bull (Tokyo) 13(5):545–550
Robaina-Rodríguez CM, Teixeira-Zullo MA, Müller-Queiróz H, de Burgos M, Alonso-Becerra E, Coll-Manchado F (2006) The preparation of the spirostanic analogues of brassinolide and castasterone. Polish J Chem 80(4):637–664
Iglesias-Arteaga MA, Pérez-Gil R, Pérez-Martínez CS, Coll-Manchado F (2001) Spirostanic analogues of teasterone. Synthesis, characterisation and biological activity of laxogenin, (23S)-hydroxylaxogenin and 23-ketolaxogenin (23-oxolaxogenin). J Chem Soc Perkin Trans 1:261–266
Fernández-Herrera MA, Sandoval-Ramírez J, López-Muñoz H, Sánchez-Sánchez L (2009) Formation of the steroidal 3β-hydroxy-6-oxo-moiety. Synthesis and cytotoxicity of glucolaxogenin. ARKIVOC 2009(13):170–184
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2013) GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC cancer base no. 11 [Internet]. Lyon, France: International Agency for Research on Cancer. http://globocan.iarc.fr. Accessed on Dec 2013
Li Y, Li Y, Li J, Pi G, Tan W (2014) Paclitaxel-and/or cisplatin-induced ocular neurotoxicity: a case report and literature review. Onco Targets Ther 7:1361–1366
Calderon-Montano JM, Burgos-Moron E, Orta ML, Lopez-Lazaro M (2014) Effect of DNA repair deficiencies on the cytotoxicity of drugs used in cancer therapy. Curr Med Chem 21(30):3419–3454
Blum KS, Pabst R (2007) Lymphocyte numbers and subsets in the human blood. Do they mirror the situation in all organs? Immunol Lett 108:45–51
Penn I (1981) Depressed immunity and the development of cancer. Clin Exp Immunol 46:459–474
Kueng W, Silber E, Eppenberger U (1989) Quantification of cells cultured on 96-well plates. Anal Biochem 182(1):16–19
Legrand C, Bour JM, Jacob C, Capiaumont J, Martial A, Marc A, Wudtke M, Kretzmer G, Demangel C, Duval D, Hache J (1992) Lactate dehydrogenase (LDH) activity of the number of dead cells in the medium of cultured eukaryotic cells as marker. J Biotechnol 25(3):231–243
Shapiro HM (1988) Practical flow cytometry, 2nd edn. Alan R. Liss, New York, pp 211–265
Lyons AB, Hasbold J, Hodgkin PD (2001) Flow cytometric analysis of cell division history using dilution of carboxyfluorescein diacetate succinimidyl ester, a stably integrated fluorescent probe. In: Darzynkiewicz Z, Robinson JP, Roederer M (eds) Essential cytometry methods. Wiley, New York, pp 375–398
Ylä-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL (2009) Monitoring autophagy by electron microscopy in mammalian cells. Methods Enzymol 452:143–164
Abramoff M, Magalhaes P, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11:36–42
Sherr CJ (2000) The Pezcoller lecture: cancer cell cycles revisited. Cancer Res 60:3689–3695
McLaughlin F, Finn P, La Thangue NB (2003) The cell cycle, chromatin and cancer: mechanism-based therapeutics come of age. Drug Discov Today 8(17):793–802
Hartwell LH, Weinert TA (1989) Checkpoints: controls that ensure the order of cell cycle events. Science 246(4930):629–634
Jackson JR, Gilmartin A, Imburgia C, Winkler JD, Marshall LA, Roshak A (2000) An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res 60(3):566–572
Li Y, Yao J, Chang M, Cuendet M, Bolton JL (2004) Altered apoptotic response in MCF 10A cells treated with the equine estrogen metabolite, 4-hydroxyequilenin. Toxicol Lett 154(3):225–233
Krajewska M, Wang HG, Krajewski S, Zapata JM, Shabaik A, Gascoyne R, Reed JC (1997) Immunohistochemical analysis of in vivo patterns of expression of CPP32 (caspase-3), a cell death protease. Cancer Res 57(8):1605–1613
Thiagarajan P, Tait JF (1990) Binding of Annexin-V placental anticoagulant proteinI to platelets—evidence for phosphatidylserine exposure in the procoagulant response of activated platelets. J Biol Chem 265:17420–17423
Tait JF, Gibson D, Fujikawa K (1989) Phospholipid binding-properties of human placental anticoagulant protein-I, a member of the lipocortin family. J Biol Chem 264:7944–7949
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J Immunol Methods 184:39–51
Loo DT (2002) TUNEL assay: an overview of techniques. In: Didenko VV (ed) In situ detection of DNA damage: methods and protocols. Humana Press, Totowa, pp 3–13
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Dunn WAJ (1994) Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol 4:139–143
Hunziker W, Simmen T, Honing S (1996) Trafficking of lysosomal membrane proteins in polarized kidney cells. Nephrologie 17:347–350
Fukuda M (1991) Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem 266:21327–21330
Seglen PO, Gordon PB (1982) 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA 79(6):1889–1892
Gordon PB, Seglen PO (1982) 6-Substituted purines: a novel class of inhibitors of endogenous protein degradation in isolated rat hepatocytes. Arch Biochem Biophys 217(1):282–294
Seglen PO, Gordon PB (1984) Amino acid control of autophagic sequestration and protein degradation in isolated rat hepatocytes. J Cell Biol 99(2):435–444
Zhao M, Ma N, Qiu F, Hai WL, Tang HF, Zhang Y, Wen AD (2014) Triterpenoid saponins from the roots of clematis argentilucida and their cytotoxic activity. Planta Med 80(11):942–948
Choi YH, Yoo DS, Cha MR, Choi CW, Kim YS, Choi SU, Lee KR, Ryu SY (2010) Antiproliferative effects of saponins from the roots of Platycodon grandiflorum on cultured human tumor cells. J Nat Prod 73(11):1863–1867
Yang CR, Zhang Y, Jacob MR, Khan SI, Zhang YJ, Li XC (2006) Antifungal activity of C-27 steroidal saponins. Antimicrob Agents Chemother 50(5):1710–1714
Tapondjou LA, Ponou KB, Teponno RB, Mbiantcha M, Djoukeng JD, Nguelefack TB, Watcho P, Cadenas AG, Park HJ (2008) In vivo anti-inflammatory effect of a new steroidal saponin, mannioside A, and its derivatives isolated from Dracaena mannii. Arch Pharm Res 31(5):653–658
Song W, Si L, Ji S, Wang H, Fang XM, Yu LY, Li RY, Liang LN, Zhou D, Ye M (2014) Uralsaponins MY, antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J Nat Prod 77(7):1632–1643
Xiao X, Zou J, Bui-Nguyen TM, Bai P, Gao L, Liu J, Liu S, Xiao J, Chen X, Zhang X, Wang H (2012) Paris saponin II of Rhizoma paridis—a novel inducer of apoptosis in human ovarian cancer cells. BioSci Trends 6(4):201–211
Wakabayashi C, Murakami K, Hasegawa H, Murata J, Saiki I (1998) An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun 246(3):725–730
Nhiem NX, Thu VK, Van Kiem P, Van Minh C, Tai BH, Quang TH, Cuong NX, Yen PH, Boo HJ, Kang JI, Kang HK, Kim YH (2012) Cytotoxic oleane-type triterpene saponins from Glochidion eriocarpum. Arch Pharm Res 35(1):19–26
Tsujimoto Y, Shimizu S (2005) Another way to die: autophagic programmed cell death. Cell Death Differ 12(Suppl 2):1528–1534
Acknowledgments
This work was supported by CONACyT Grants 180526, 176858, and 176863. We thank PAPIIT IN222114, VIEP-BUAP, and CUVyTT-BUAP for academic and financial support. This paper constitutes a partial fulfilment of the Doctorado en Ciencias Médicas y Biológicas of the Universidad Autónoma Benito Juárez de Oaxaca, México. The work in Mérida is supported by the L’Oréal-UNESCO For Women in Science program, the Mexican Academy of Science and CONACyT. The authors would like to thank Paul C. Kersey Johnson for reviewing the English word usage and grammar.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sánchez-Sánchez, L., Escobar, M.L., Sandoval-Ramírez, J. et al. Apoptotic and autophagic cell death induced by glucolaxogenin in cervical cancer cells. Apoptosis 20, 1623–1635 (2015). https://doi.org/10.1007/s10495-015-1181-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1181-6