Abstract
Non-adherence remains the largest cause of treatment failure to antiretroviral therapy (ART). Despite having the largest HIV pandemic, few successful adherence interventions have been conducted in South Africa. Active visualisation is a novel intervention approach that may help effectively communicate the need for consistent adherence to ART. The current study tested an active visualisation intervention in a sample of non-adherent patients. 111 patients failing on first- or second-line ART were recruited from two sites in the Western Cape, South Africa. Participants were randomly allocated to receive the intervention or standard care (including adherence counselling). The primary outcome was adherence as measured by plasma viral load (VL). There was a clinically significant difference (p = 0.06) in VL change scores between groups from baseline to follow-up, where the intervention had a greater decrease in log VL (Madj = − 1.92, CI [− 2.41, − 1.43), as compared to the control group (Madj = − 1.24, [− 1.76, − 0.73]). Participants in the intervention group were also significantly more likely to have a 0.5 log improvement in VL at follow-up (\(\chi_{(1)}^{2}\) = 4.82, p = 0.028, ɸ = 0.28). This study provides initial evidence for the utility of this novel, brief intervention as an adjunct to standard adherence counselling, for improving adherence to ART.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of antiretroviral therapy (ART) has revolutionised HIV from a once fatal infection into a manageable chronic illness. Despite the ability of ART to maintain virological suppression and prevent clinical progression, non-adherence remains the prevalent reason for treatment failure [1], and therefore a critical area for intervention.
South Africa has the largest global HIV pandemic. In 2017, the estimated countrywide prevalence rate was 12.6%, and the total number of South Africans living with HIV was 7.06 million. This estimated prevalence is even greater (18%) among those aged 15–49 years [2]. While disparity in treatment availability remains a substantial contextual issue for healthcare, it is also vital to prevent treatment failure within those patients who do have access to care.
Non-adherence, as opposed to resistance, is regarded as the most significant reason for treatment failure for patients living with HIV in South Africa [3]. Few successful adherence interventions for ART have been conducted within South Africa. Recent meta-analyses of interventions which included South African samples found that enhanced standard of care (adherence counselling) supplemented with text-messaging or treatment supporters produced distinguishable improvements in adherence as compared to standard care [4, 5]. The South African studies included here however did not report consistent objective changes in adherence from the interventions delivered [6, 7]. Overall, the interventions that do create change have small effects, which decrease when the intervention is withdrawn [5].
A significant issue highlighted by these meta-analyses is the variation in the measurement of adherence as an outcome. Many studies rely on self-reported adherence, which may overestimate the positive effects found. While there is no “gold standard” adherence measure for HIV [8], viral load (VL) as measured through plasma provides an objective biological marker of treatment adherence. Using VL as a measure can therefore negate some of the uncertainty around actual medication-taking behaviour when using self-report, pill counts, prescription refills, or other proxy measures. Although collecting blood test data may be more laborious than other measures of adherence, VL provides an objective way to evaluate the effectiveness of adherence interventions on clinical progression.
Active visualisation is a method of delivering health information that could be particularly useful in educating patients about ART. Patients with HIV may discontinue treatment due to a lack of change in symptoms experienced [9], and additionally may not perceive immediate consequences of increased symptoms from non-adherence. Active visualisation can educate and motivate patients to adhere by helping them to understand the processes occurring inside their body and the purpose of their treatment. Active visualisation uses dynamic visual representations, such as animations, computer modelling, or physical demonstrations to present information about internal bodily processes of disease and treatment mode-of-action [10].
A previous study found that incorporating active visualisation into a smartphone application improved adherence to ART as compared to medication reminders alone [11]. Participants received either a standard application with reminders only, or an augmented version including a simulation of VL and CD4 cell activity, based on self-reported adherence. The augmented application group self-reported greater adherence and had reduced VL at 3-month follow-up, as compared to the reminder only group. Although limited by a small sample size, this study demonstrates the additive effect that active visualisation may have on adherence to ART.
A physical demonstration of the physiological treatment process may be the most appropriate form of active visualisation to use within the South African context. This format does not rely on access to technology, yet is still portable and easy to disseminate. An example physical demonstration has been found to improve understanding and treatment efficacy beliefs of phosphate binding medication in end stage renal disease patients [12].
A similar physical demonstration has been created to visually demonstrate the effects of adherence or non-adherence to ART [10]. This device presents an innovative way to portray the importance of consistent adherence, a message relevant to patients failing first- and second-line treatment and is a concept often misunderstood by patients [13]. A visual depiction of VL and treatment mode-of-action may provide a more salient representation of the effects of treatment failure, in contrast to standard verbal information the patient may have already received. The current study was a randomised controlled trial assessing the efficacy of an active visualisation device in improving adherence to ART, within a sample of non-adherent patients in Western Cape, South Africa.
Method
Participants
Study participants were patients living with HIV, attending two separate infectious diseases clinics in Western Cape, South Africa. Both clinics provide basic non-specialized hospital services and offer full ART services as part of their outpatient services. Patients are referred from general services to the clinics following an HIV positive test result. The ART programmes enrol new patients on a continuous basis and provide regular patient care, blood tests, and ART medication refills to patients. The areas served by the clinics have wide socio-economic disparities, and levels of poverty and unemployment are high.
Due to the large variability of effect sizes found in previous adherence intervention studies, we choose to detect a medium effect size only. A G * Power [14] calculation revealed that 128 participants would be necessary using a two-tailed test, with 80% power and a significance level of 0.05. We were able to recruit a total of 111 patients, which included 78 participants from the Infectious Diseases Clinic at a major peri-urban hospital between May and November 2016, and a further 33 patients from a community clinic between August and October 2017.
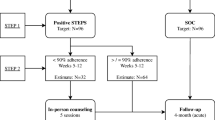
Patients were included in the study if they were failing on first- or second-line treatment, over 15 years of age, and fluent in either English or Afrikaans. Treatment failure was classified as having been on ART for 4 months and having a baseline VL of more than 1000 copies/mL. Clinical staff referred patients to the research assistant for recruitment based on this eligibility criteria for treatment failure. Informed consent or assent was obtained from all participants included in the study. Figure 1 depicts the flow of participants through the study.
Procedure
Patients were recruited after their appointment with the treating clinician. All patients recruited into the study were receiving standard care. Standard care for patients with a raised VL within these treatment settings includes adherence counselling from the treating clinician and a discussion of potential causes of their sub-optimal adherence during their appointment. If patients continue to default, they receive monthly consultations with the doctor and counsellor.
During recruitment, the treating clinician at each clinic identified patients who fit study criteria. Following the consultation, the research assistant met interested patients in a separate counselling room. The research assistant discussed the participant information sheet with the patient and obtained informed consent. After participants completed the baseline questionnaire, an opaque sealed envelope was opened containing their randomisation allocation into one of two study groups (control n = 55, intervention n = 56). These envelopes were created by one of the researchers (AJ) independent to the recruitment process, using a random number sequence generator. This concluded the study session for those assigned to the control group. Participants in the intervention group were shown the active visualisation demonstration (details below). Following the intervention, participants completed the post-intervention questionnaire before concluding the study session and their contact with the research assistant. The research assistant collected follow-up blood test data from patient medical files.
Intervention
The intervention was an active visualisation device developed by the study authors (AJ, KP, EC, & MT). The intervention was delivered by the research assistants who were trained by one of the researchers (AJ). The intervention took approximately 10 minutes with each participant.
The intervention demonstrates the importance of consistent adherence to ART by symbolising the treatment mode-of-action within a ‘body’. The body-shaped container was created using two pieces of laser cut Perspex (total height = 32.5 cm, total length = 40.5 cm, and width = 2.4 cm) and waterproof sealant. When the ART ‘medication’ (an aspirin tablet) is added to the body, the liquid inside changes colour from pink (indicating the presence of the virus) to clear (representing medication controlling viral replication). The change in colour occurs through manipulating the pH balance of the liquid inside using an acid (aspirin tablet) versus a basic solution (sodium hydroxide).
The intervention runs through scenarios to demonstrate that medication must be taken each day, as even when medication is added to the ‘body’, the following day the pink colour returns because the infection cannot be reversed. This process demonstrates why consistent adherence is needed to achieve virological suppression. The intervention also demonstrates the effects of missing one or two doses of ART, versus how long-term non-adherence can lead to treatment failure. Detailed methodology of the device, including pictures and a video of the device in use, can be found in the supplementary material of our previously published editorial [10].
Measures
Demographic and Clinical Information
Demographic variables were self-reported in the baseline questionnaire, including age, gender, ethnicity, highest level of education, and approximate monthly family income. Clinical information was obtained from patient medical records regarding current medication regimen (first- or second-line treatment) and date of diagnosis.
Viral Load
The primary outcome of the study was adherence as measured by plasma VL. The amount of virus within each participant’s plasma was measured using the COBAS AmpliPrep/Taqman HIV-1 test (Roche Laboratories, California), a reverse transcription polymerase chain reaction test with the capacity to quantitate HIV-1 RNA over the range of 20–10,000,000 copies per mL. Baseline VL data was the most recent, available VL from no earlier than 5 months prior to study session for each participant (M = 40.92 days, SD = 37.41 days). Follow-up VL data was included from at least 2 months post study session, with the latest data point being 16.2 months later (M = 266.43 days, SD = 121.66 days). Data were excluded from the analysis if baseline VL was less than 1000 copies/mL (n = 5). Figure 1 details the number of participants included in the final analysis.
Illness Perceptions, Medication Beliefs, and Other Perceptions
Illness perceptions, beliefs about medication, and other perceptions were measured at baseline and post-intervention. The Brief Illness Perception Questionnaire (Brief IPQ) [15] asks participants to rate cognitive and emotional representations of their illness (“HIV infection”), on an 11-point, Likert-type scale from 0 to 10 with relevant anchors. Beliefs about medication were assessed using two items based on the necessity-concerns framework [16]. One item asks participants how much they feel they need their medication (necessity belief); the other asks how concerned they are about long-term effects of their medication (concern belief). Both items are answered on an 11-point Likert-type scale with relevant anchors.
Additional items asked participants how motivated they were to take their ART medication, how serious of an illness they think HIV is, how difficult they find taking their HIV treatment, and their perceived risk of HIV developing into a more serious illness. Each of these items were rated on an 11-point, Likert-type scale from 0 to 10, with relevant anchors for each (e.g. “Not at all serious” versus “Extremely serious”).
Depressive Symptoms
Symptoms of depression were measured at baseline using the Patient Health Questionnaire 9 [17], due to the known relationship between depression and adherence in HIV [18]. The PHQ9 is a 10-item measure assessing frequency (from “not at all” to “nearly every day”) of depressive symptomology over the past 2 weeks. The total score of the first nine-items was used in the current study.
Evaluation of the Visualisation Device
Participants also completed six items in the post-intervention questionnaire evaluating their thoughts about the visualisation device. Participants rated the device on how interesting it was, how motivated it made them to take their medication, how it helped their understanding of HIV and their medication, and how anxious it made them about HIV and their medication. These ratings were made on an 11-point Likert-type scale from 0 to 10 with relevant anchors for each item.
Finally, an open-ended item asked participants “What thoughts ran through your mind when you were shown the device?”. This question has been used previously in visualisation research [19]. Four main response categories were determined. Adherence thoughts were any thoughts relating to medication taking, concerns were anxieties about the device or personal health, and education thoughts were those stating an improved understanding after seeing the device. Irrelevant comments (e.g. “pay attention”) were coded into a fourth “other” category. Two independent raters coded whether each response fit into one or more categories, with a third coder making the final decision regarding discrepancies.
Statistical Analysis
The data were analysed using SPSS version 25.0. Tests were considered significant if a significance value below 0.05 was achieved. Independent samples t tests and Chi square tests assessed differences at baseline in demographic, clinical, and outcome variables between groups. Paired-samples t tests were used to assess differences within the intervention group from baseline to post-intervention in illness perceptions, medication beliefs, and other beliefs. One-way, between groups ANCOVAs were used to assess differences between groups in change scores in raw and log VL from baseline to follow-up, controlling for baseline VL. A Chi square test examined the association between group and whether there was an improvement in log VL of 0.5 or more from baseline to follow-up (yes/no).
Results
Demographic and Clinical Variables
Participants ranged from 15 to 59 years of age (M = 36.48, SD = 9.66), were mainly female (75/11, 67.6%), and identified as African (75/111, 67.6%) or Coloured (34/11, 30.6%) (see Table 1 for sample characteristics). There were no baseline differences found in any demographic or outcome variables between groups (p > 0.05). Baseline levels of depressive symptoms were not correlated with change in VL (r = − 0.03, p = 0.775) or change in log VL scores (r = 0.01, p = 0.929). There was no significant association between medication regimen and group (see Table 1), with most participants on first-line treatment (83/107, 77.6%). Length of diagnosis ranged from 7.24 months to 20.68 years prior to recruitment (M = 6.81 years, SD = 4.56 years).
Baseline differences in demographic and clinical variables between the two treatment sites were also assessed. There were no significant differences between participants from each treatment site in demographic variables (p > 0.05). All clinical variables between sites were also not significantly different (p > 0.05) except for the amount of days between study session and follow-up, where participants from the peri-urban hospital had a significantly greater number of days before follow-up (M = 281.46, SD = 114.28), than participants from the community clinic (M = 148.73, SD = 106.13; t(67) = 4.04, p < 0.001).
Illness Perceptions, Medication Beliefs, and Evaluation of Visualisation Device
There were no significant changes in illness perceptions, medication beliefs, motivations to take treatment, seriousness beliefs, perceived risk, or treatment difficulty in the intervention group from baseline to post-intervention (p > 0.05). Perceptions of treatment control (M = 8.91, SD = 2.14), illness understanding (M = 8.48, SD = 2.62), and medication necessity beliefs (M = 8.71, SD = 2.77) were high at baseline (maximum score for each item is 10).
Intervention participants rated the device highly, finding it helpful in explaining their HIV infection (M = 9.5, SD = 1.56) and medication (M = 9.32, SD = 1.87), interesting (M = 9.55, SD = 1.40), and motivating regarding taking their medication (M = 9.33, SD = 1.16). The device made participants moderately anxious about their HIV (M = 6.80, SD = 3.79) and medication (M = 6.23, SD = 4.20). Participants’ open-ended thoughts about the device most commonly fit into the adherence category (44.6%, 31/56), followed by education (41.1%, 23/56), and concern thoughts (19.6%, 11/56). Other thoughts comprised 23.3% of responses (13/56).
Adherence
Of the 111 participants recruited at baseline, 103 (92.8%) had a baseline VL, and 86 had a follow-up VL (77.5%). Only 84 participants in the sample (75.7%) had both a baseline and follow-up VL. Additional information regarding data excluded from analyses can be found in Fig. 1. There were no demographic differences between participants who did, and did not have a follow-up VL (p > 0.05). There were no differences in baseline VL between participants with a baseline VL only, versus the rest of the sample (p > 0.05). There was also no difference in VL change scores between participants attending the community clinic versus the peri-urban hospital (p = 0.198).
An ANCOVA controlling for baseline VL assessed group differences in VL change scores from baseline to follow-up. There was a significant difference in change in raw VL between groups (F(1, 58) = 5.33, p = 0.025, partial ƞ2 = 0.08), where the intervention group had a significantly greater decrease in VL at follow-up (Madj = − 76,961.47, CI [− 100,099.81, − 53,823.13]), compared to the control group (Madj = − 37,836.03, CI [− 62,166.48, − 13,505.59]) (see Fig. 2). A second ANCOVA assessed group differences in log VL change scores between groups, controlling for baseline log VL. Although the intervention group had a greater decrease in log VL at follow-up (Madj = − 1.92, CI [− 2.41, − 1.43), compared to the control group (Madj = − 1.24, CI [− 1.76, − 0.73]), this difference was not statistically significant (F(1, 58) = 3.67, p = 0.06, partial ƞ2 = 0.06) (see Fig. 3). This group difference, however, can be regarded as clinically significant, as a change in log VL of 0.5 or greater has been identified as a reduction of clinical significance [20, 21]. Each decrease of 0.5 in log VL is associated with a 30% reduction in the risk of clinical progression.
Based on the analysis used in a previous intervention trial [21], we created a dichotomous variable to assess the association between group and whether an improvement in log VL of 0.5 or greater had occurred between baseline and follow-up. There was a significant association between group and improvement in 0.5 log VL or greater (\(\chi_{(1)}^{2}\) = 4.82, p = 0.028, ɸ = 0.28), with more participants in the intervention group demonstrating this improvement (26/42, 61.9%), as compared to the control group (16/42, 38.1%). There were also more participants who did not demonstrate an improvement of 0.5 or more in log VL in the control group (13/19, 68.4%), as compared to the intervention group (6/19, 31.1%).
Discussion
This is the first trial investigating the utility of an active visualisation intervention for improving adherence to ART. The results of the trial provide preliminary evidence for the efficacy of this brief intervention in improving adherence for patients failing first- or second-line ART, as demonstrated by a greater improvement in VL.
This brief intervention resulted in a clinically significant improvement in adherence, when compared to those receiving standard care only. Furthermore, participants in the intervention group were significantly more likely to have a 0.5-log improvement in VL at follow-up. It is important to note that standard care in this context includes adherence counselling. As described above, this includes the treating clinician consulting with patients regarding the cause of their non-adherence and the risk of clinical progression, which may continue monthly if issues persist. It is therefore unsurprising that we see improvements across time in both groups, but also significant that this brief visualisation intervention was able to produce a greater improvement in adherence over and above that achieved through standard adherence counselling. This suggests that the intervention could be a worthwhile adjunct to standard care within this context. Our results also support the findings of Perera and colleagues [11] and the use of active visualisation as a method for educating and motivating patients to improve adherence to ART.
Previous intervention studies conducted with HIV patients have relied on the use of counselling and treatment supporters [5, 22] or directly observed therapy [23]. These forms of intervention require extensive time commitments, training, and readily available support throughout. Furthermore, the small effects found within these trials can dissipate after the withdrawal of the intervention. The current intervention therefore presents an innovative, brief approach that can easily be added to standard care or incorporated into other adherence interventions.
Interestingly, we found no changes in illness perceptions or medication beliefs in participants who saw the visualisation device. This finding was surprising as these changes are often found in visualisation studies, particularly in regard to perceptions such as treatment control and understanding [19, 24, 25]. In the current study however, participants did report high perceptions of treatment control, understanding, and necessity medication beliefs at baseline, meaning a ceiling effect may have occurred.
Participants appeared to be accepting of the intervention, rating the device as easy to understand, interesting, and motivating in regards to taking their medication. There were moderate levels of anxiety reported upon seeing the intervention. This is understandable as visualisation exposes the participant to the real threat of possible treatment failure. Combining this message with the information regarding treatment efficacy may have motivated participants to adhere to treatment, rather than making them avoidant due to raised anxiety. The open-ended question data suggested that the majority of participants understood the key message of consistent adherence in preventing treatment failure.
Finally, the study findings also support the use of visualisation as an alternate format for delivering health information. Participants had likely been exposed to multiple explanations regarding ART. Perhaps the unique visual delivery of this information increased salience and therefore motivation for these participants. The results also suggest that visualisation can improve health outcomes. These results are promising and promote the use of visualisation as an education tool for other illnesses and treatments.
Our study is limited by the lack of available VL data in our recruited sample and the variability in time between recruitment and follow-up VL data. This was due to an absence of routine monitoring and clinical data available within the treatment context of an overburdened health care system. Alternatively, some patients had to be excluded from the analysis who were referred by clinical staff and recruited into the study, but were ineligible due to low baseline VL (< 1000) or having no available baseline VL data. Research assistants did not have access to patient data at the time of recruitment, meaning they relied upon referrals from clinical staff to ensure patients were non-adherent and could be included. This could have been avoided by requesting an independent blood test as part of the study, however knowledge of this monitoring may also have impacted adherence. Second, we did not have clear information regarding other medical issues that may affected VL (such as alcohol use or breastfeeding), and were therefore unable to control for the impact of these variables. Third, we did not have information regarding resistance from the blood tests within our sample, meaning we are unable to assume that differences in VL are exclusively the result of changes in treatment adherence.
Another limitation to consider is the significantly shorter follow-up VL data available from the community clinic sample as compared to the peri-urban hospital sample. This could suggest that patients recruited from the community clinic were more adherent in terms of attending their appointments and providing blood samples, or alternatively, that this clinic kept better records than the peri-urban hospital. There were however no differences found between sites in VL change scores, suggesting that differences in the data available may not necessarily reflect differences in patient adherence. Finally, the reduced sample size with available VL data at baseline and follow-up decreased the statistical power in our analysis. This reduces the generalisability of our study findings. However, as stated above, analyses established that there were no demographic differences between participants who did and did not have follow-up VL, suggesting that the sample analysed was comparable to those recruited.
This study does however have significant strengths, including the use of a randomised controlled design, an objective measure of an adherence marker, and good ecological validity in a high-need population. The intervention has high clinical applicability and could be easily integrated into standard clinical care, as an adjunct to adherence counselling. The portability and low-cost of the device mean it could be utilised in hard to reach populations with limited access to healthcare and technology. This could overcome the geographic limitations of previous interventions such as cognitive behavioural therapy and counselling, which rely on access to trained health professionals [5]. The simple message of the intervention may also translate well to adolescents with HIV in South Africa, a sub-population with significant adherence issues [26, 27]. The intervention could also promote adherence to newly diagnosed patients at treatment initiation, and potentially negate the occurrence of non-adherence.
Conclusion
The current study provides initial evidence that active visualisation may be an effective intervention for improving adherence to ART. This novel intervention presents an exciting opportunity for an alternative intervention strategy that is brief and could easily be added to adherence counselling or other adherence interventions in clinical practice. Further research could investigate the utility of the device in additional HIV patient populations, including remote populations, newly diagnosed patients, and adolescents.
References
Conway B. The role of adherence to antiretroviral therapy in the management of HIV infection. J Acquir Immune Defic Syndr. 2007;45:S14–8.
Statistics South Africa. Mid-year population estimates. 2017.
Murphy RA, Sunpath H, Castilla C, Ebrahim S, Court R, Nguyen H, et al. Second-line antiretroviral therapy: long-term outcomes in South Africa. J Acquir Immune Defic Syndr. 2012;61(2):158–63.
Mills EJ, Lester R, Thorlund K, Lorenzi M, Muldoon K, Kanters S, et al. Interventions to promote adherence to antiretroviral therapy in Africa: a network meta-analysis. Lancet HIV. 2014;1(3):e104–11.
Kanters S, Park JJ, Chan K, Socias ME, Ford N, Forrest JI, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV. 2017;4(1):e31–40.
Peltzer K, Ramlagan S, Jones D, Weiss SM, Fomundam H, Chanetsa L. Efficacy of a lay health worker led group antiretroviral medication adherence training among non-adherent HIV-positive patients in KwaZulu-Natal, South Africa: results from a randomized trial. SAHARA-J: J Soc Asp HIV/AIDS. 2012;9(4):218–26.
Nachega JB, Chaisson RE, Goliath R, Efron A, Chaudhary MA, Ram M, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS. 2010;24(9):1273–80.
Mugavero MJ, Westfall AO, Zinski A, Davila J, Drainoni ML, Gardner LI, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61(5):574–80.
Dahab M, Charalambous S, Hamilton R, Fielding K, Kielmann K, Churchyard GJ, et al. “That is why I stopped the ART”: patients’ and providers’ perspectives on barriers to and enablers of HIV treatment adherence in a South African workplace programme. BMC Public Health. 2008;8(1):63.
Jones AS, Petrie KJ. I can see clearly now: using active visualisation to improve adherence to ART and PrEP. AIDS Behav. 2017;21(2):335–40.
Perera AI, Thomas MG, Moore JO, Faasse K, Petrie KJ. Effect of a smartphone application incorporating personalized health-related imagery on adherence to antiretroviral therapy: a randomized clinical trial. AIDS Patient Care STDs. 2014;28(11):579–86.
Karamanidou C, Weinman J, Horne R. Improving haemodialysis patients’ understanding of phosphate-binding medication: a pilot study of a psycho-educational intervention designed to change patients’ perceptions of the problem and treatment. Br J Health Psychol. 2008;13(2):205–14.
Kagee A, Remien R, Berkman A, Hoffman S, Campos L, Swartz L. Structural barriers to ART adherence in Southern Africa: challenges and potential ways forward. Glob Public Health. 2011;6(1):83–97.
Faul F, Erdfelder E, Lang A, Buchner A. G * Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91.
Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–7.
Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLoS ONE. 2013;8(12):e80633.
Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–7.
Jones AS, Fernandez J, Grey A, Petrie KJ. The impact of 3-D models versus animations on perceptions of osteoporosis and treatment motivation: a randomised trial. Ann Behav Med. 2017;51(6):899–911.
Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4 lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126(12):946–54.
Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46(4):443–50.
Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438.
Nachega JB, Chaisson RE, Goliath R, Efron A, Chaudhary MA, Ram M, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS. 2010;24(9):1273–80.
Jones AS, Ellis CJ, Nash M, Stanfield B, Broadbent E. Using animation to improve recovery from acute coronary syndrome: a randomized trial. Ann Behav Med. 2016;50(1):108–18.
Stephens MH, Grey A, Fernandez J, Kalluru R, Faasse K, Horne A, et al. 3-D bone models to improve treatment initiation among patients with osteoporosis: a randomised controlled pilot trial. Psychol Health. 2016;31(4):487–97.
Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65–71.
Nglazi MD, Kranzer K, Holele P, Kaplan R, Mark D, Jaspan H, et al. Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC Infect Dis. 2012;12(1):21.
Acknowledgements
The authors would like to thank the participants and clinical staff who helped with recruitment. We would also like to acknowledge Professor Mary Jane Rotheram and Professor Mark Tomlinson for their support of this project.
Funding
This project was supported by funding from the Partnership for Alcohol and AIDS Intervention Research (PAAIR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Ethics approval was received from Stellenbosch University Health Research Ethics Committee (#N16/01/003). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Jones, A.S.K., Coetzee, B., Kagee, A. et al. The Use of a Brief, Active Visualisation Intervention to Improve Adherence to Antiretroviral Therapy in Non-adherent Patients in South Africa. AIDS Behav 23, 2121–2129 (2019). https://doi.org/10.1007/s10461-018-2292-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-018-2292-1