Abstract
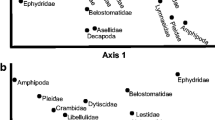
Despite their widespread use in grazer–biofilm studies, stream exclusion cages have inherent physical properties that may alter benthic organism colonization and growth. We used laboratory studies and a field experiment to determine how exclusion cage design (size and material) alters light availability, water velocity, and benthic organism colonization. We measured light reduction by various plastic cage materials and flow boundary layer thickness across a range of exclusion cage sizes in the laboratory. We also deployed multiple exclusion cage designs based on commonly available materials into a second-order stream to assess algae and macroinvertebrate colonization differences among exclusion cages. All plastics reduced some light (190–700 nm wavelengths) and blocked more ultraviolet light than photosynthetically active radiation. Exclusion cage size did not influence flow boundary layer thickness, but larger exclusions tended to have higher velocity at the substrata surface. Despite light and water velocity differences, algal biomass, macroinvertebrate density, and community composition were similar between exclusion cage types. However, algal assemblages outside exclusion cages differed in composition and had higher biomass compared to inside exclusion cages. In terms of algal and macroinvertebrate colonization, plastic exclusion cage size and material appear to be flexible within the sizes tested, but differences can still exist between exclusion cage communities and those within the stream. Overall, artifacts of screened exclusion cages do not appear to introduce large bias in results of grazer–biofilm studies, but efforts to design exclusion cages that better mimic the natural system should continue.




Similar content being viewed by others
References
Albertson LK, Daniels MD (2016) Effects of invasive crayfish on fine sediment accumulation, gravel movement, and macroinvertebrate communities. Freshw Sci 35:644–653
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, DC
Bailey RC, Norris RH, Reyndolson TB (2001) Taxonomic resolution of benthic macroinvertebrate communities in bioassessments. J N Am Benthol Soc 20:280–286
Baker SC (1983) Morphometry, physico-chemical parameters, and benthic macroinvertebrate structure as a function of stream order in the Blackburn Fork drainage in Putnam and Jackson Counties, Tennessee. MS thesis, Tennessee Tech University, Tennessee
Bertrand KN, Gido KB (2007) Effects of the herbivorous minnow, southern redbelly dace (Phoxinus erythrogaster), on stream productivity and ecosystem structure. Oecologia 151:69–81
Biggs BJF, Goring DG, Nikora VI (1998) Subsidy and stress responses of stream periphyton to gradients in water velocity as a function of community growth form. J Phycol 34:598–607
Brown GG, Norris RH, Maher WA, Thomas K (2000) Use of electricity to inhibit macroinvertebrate grazing of epilithon in experimental treatments in flowing waters. J N Am Benthol Soc 19:176–185
Carling PA (1992) The nature of the fluid boundary layer and the selection of parameters for benthic ecology. Freshw Biol 28:273–284
Cattaneo A, Keimian T, Roberge M, Marty J (1997) Periphyton distribution and abundance on substrata of different size along a gradient of stream trophy de Montréal. Hydrobiologia 354:101–110
Charpentier B, Morin A (1994) Effect of current velocity on ingestion rates of black fly larvae. Can J Fish Aquat Sci 51:1615–1619
Connolly NM, Pearson RG (2013) Nutrient enrichment of a heterotrophic stream alters leaf litter nutritional quality and shredder physiological condition via the microbial pathway. Hydrobiologia 718:85–92
Cooper SD, Walde SJ, Peckarsky BL (1990) Prey exchange rates and the impact of predators on prey populations in streams. Ecology 71:1503–1514
Dodds WK, Biggs BJF (2002) Water velocity attenuation by stream periphyton and macrophytes in relation to growth form and architecture. J N Am Benthol Soc 21:2–15
Donato-Rondón JC, Morales-Duarte SJ, Castro-Rebolledo MI (2010) Effects of eutrophication on the interaction between stream algae and grazers in an Andean stream. Hydrobiologia 657:159–166
Douglas M, Lake PS (1994) Species richness of stream stones: an investigation of the mechanisms generating the species-area relationship. Oikos 69:387–396
Edington JM (1968) Habitat preferences in net-spinning caddis larvae with special reference to the influence of water velocity. J Anim Ecol 37:675–692
Erdem N, Erdogan UH, Cireli AA, Onar N (2010) Structural and ultra-violet protective properties of nano-TiO2-doped polypropylene filaments. J Appl Polym Sci 115:152–157
Feminella JW, Hawkins CP (1995) Interactions between stream herbivores and periphyton: a quantitative analysis of past experiments. J N Am Benthol Soc 14:465–509
Grossman AR, Bhaya D, Apt KE, Kehoe DM (1995) Light-harvesting complexes in oxygenic photosynthesis: diversity, control, and evolution. Ann Rev Genet 29:231–288
Häder DP, Kumar HD, Smith RC, Worrest RC (2007) Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochem Photobiol Sci 6:267–285
Hart DD, Merz RA (1998) Predator–prey interactions in a benthic stream community: a field test of flow-mediated refuges. Oecologia 114:263–273
Hauxwell J, McClelland J, Behr PJ, Valiela I (1998) Relative importance of grazing and nutrient controls of macroalgal biomass in three temperate shallow estuaries. Estuaries 21:347–360
Henderson K (2015) The association among water depth, algae, and hypoxia in agricultural lakes. MS thesis, Tennessee Tech University, Tennessee
Hill WR (1996) Factors affecting benthic algae: effects of light. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic, London, pp 121–148
Hill WR, Knight AW (1987) Experimental analysis of the grazing interaction between a mayfly and stream algae. Ecology 68:1955–1965
Hillebrand H, Kahlert M (2001) Effect of grazing and nutrient supply on periphyton biomass and nutrient stoichiometry in habitats of different productivity. Limnol Oceanogr 46:1881–1898
Hintz WD, Wellnitz T (2013) Current velocity influences the facilitation and removal of algae by stream grazers. Aquat Ecol 47:235–244
Horner RR, Welch EB (1981) Stream periphyton development in relation to current velocity and nutrients. Can J Fish Aquat Sci 38:449–457
Malmqvist B (1993) Interactions in stream leaf packs: effects of a stonefly predator on detritivores and organic matter processing. Oikos 1:454–462
Moulton TP, de Souza ML, Silveira RML, Krsulović FAM (2004) Effects of ephemeropterans and shrimps on periphyton and sediments in a coastal stream (Atlantic forest, Rio de Janeiro, Brazil). J N Am Benthol Soc 23:868–881
Murdock JN, Gido KB, Dodds WK, Bertrand KN, Whiles MR (2010) Consumer return chronology alters recovery trajectory of stream ecosystem structure and function following drought. Ecology 91:1048–1062
Odum EP, Finn JT, Franz EH (1979) Perturbation theory and the subsidy-stress gradient. Bioscience 29:349–352
Patrick RH (1967) The effect of invasion rate, species pool, and size of area on the structure of the diatom community. Proc Natl Acad Sci 58:1335–1342
Peckarsky BL, Penton MA (1990) Effects of enclosures on stream microhabitat and invertebrate community structure. J N Am Benthol Soc 9:249–261
Perrin CJ, Wilkes B, Richardson JS (1992) Stream periphyton and benthic insect responses to additions of treated acid mine drainage in a continuous-flow on-site mesocosm. Environ Toxicol Chem 11:1513–1525
Poff NLR, Ward JV (1992) Heterogeneous currents and algal resources mediate in situ foraging activity of a mobile stream grazer. Oikos 65:465–478
Power ME, Matthews WJ, Stewart AJ (1985) Grazing minnows, piscivorous bass, and stream algae: dynamics of a strong interaction. Ecology 66:1448–1456
Pringle CM, Blake GA (1994) Quantitative effects of atyid shrimp (Decapoda: Atyidae) on the depositional environment in a tropical stream: use of electricity for experimental exclusion. Can J Fish Aquat Sci 51:1443–1450
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Rosemond AD, Mullholland PJ, Elwood JW (1993) Top-down and bottom-up control of stream periphyton: effects of nutrients and herbivores. Ecology 74:1264–1280
Sartory DP, Grobbelaar JU (1984) Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114:177–187
Scheder C, Waringer JA (2002) Distribution patterns and habitat characterization of Simuliidae (Insecta: Diptera) in a low-order sandstone stream (Weidlingbach, lower Austria). Limnologica 32:236–247
Slack WT, O’Connell MT (1998) Design for a portable, modular enclosure/exclosure device. J Freshw Ecol 13:193–206
Steinman AD (1996) Effects of grazers on freshwater benthic algae. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic, London, pp 341–373
Tao Y, Zhang X, Au DWT, Mao X, Yuan K (2010) The effects of sub-lethal UV-C irradiation on growth and cell integrity of cyanobacteria and green algae. Chemosphere 78:541–547
Vogel S (1981) Life in moving fluids. Princeton University Press, Princeton
Vogel S (1994) Life in moving fluids, 2nd edn. Princeton University Press, Princeton
Way CM, Burky AJ, Bingham CR, Miller AC (1995) Substrate roughness, velocity refuges, and macroinvertebrate abundance on artificial substrate in the lower Mississippi River. J N Am Benthol Soc 14:510–518
Wellnitz T (2014) Can current velocity mediate trophic cascades in a mountain stream? Freshw Biol 59:2245–2255
Woodward G, Papantoniou G, Edwards F, Lauridsen R (2008) Trophic trickles and cascades in a complex food web: impacts of a keystone predator on stream community structure and ecosystem processes. Oikos 117:683–692
Zimmerman JKH, Vondracek B (2006) Effects of stream enclosures on drifting invertebrates and fish growth. J N Am Benthol Soc 25:453–464
Acknowledgements
This study was funded by the School of Environmental Studies and Biology Department at Tennessee Tech University. Thanks to Kelly Dunham, Kristi Catignani, and Tori Kauffman for their help in the field and laboratory, to Jason Payne for nutrient analyses, and to Walter Dodds for use of the thermistor water velocity sensor.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Piet Spaak
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Knorp, N.E., Murdock, J.N. Exclusion size and material have minimal effects on stream benthic algae and macroinvertebrate colonization within submerged cages. Aquat Ecol 51, 545–556 (2017). https://doi.org/10.1007/s10452-017-9635-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-017-9635-2