Abstract
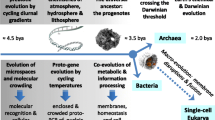
It is often assumed that the transition between chemical evolution and biological evolution undergoes a smooth process; that once life has arisen, it will automatically ‘flood’ a solar system body. However, there is no a priori reason to assume that a link between them is a given. The fact that both chemical evolution and biological evolution meet in a single point can be critical. Thus, one may ask: can a world’s environment be favourable for chemical evolution but not for biological evolution, or vice versa? This is an important question worth exploration because certain worlds in the solar system in the past seemed to possess the possibility of chemical evolution, while several worlds in the present seem to exhibit such a possibility. Have such solar system bodies thus been, or are, ‘flooded’ by life? Did they possess the opportunity for biological evolution? The answer depends on the very nature of certain conditions under which evolution occurs, which may indicate that a link between chemical evolution and biological evolution is not automatically realised on a habitable solar system body. Thus, these conditions imply that in the emergence and distribution of cellular life, there exists an indeterminacy bottleneck at which chemical evolution and biological evolution meet through a single cell, whose descendants goes ‘information explosive’, ‘entropy implosive’ and ‘habitat expansive’, which determine whether life moves on to new environments. The consequence is that a world's environment can indeed be favourable for biological evolution, but not for chemical evolution. Thus, even if chemical evolution leads to the emergence of a microbial organism in a world, then it is not a given that such a first life form will be subjected to distribution to other environments; and not a given that its existence will continue in the environment it originated in. Thus, the bottleneck may be one of the decisive factors in the differences between habitable and inhabited worlds.


Similar content being viewed by others
Notes
An inverse proportionality is not necessarily observed in the flask of biological evolution. Although it is widely believed that single-celled organisms necessarily evolve into multi-cellular organisms, this trend is not observed in the history of life on the Earth; in Darwinian evolution, there are no lower or higher organisms, there is only reproductive success. Thus, microbial life represents the majority of life established in the past and present and will probably continue to represent the majority of life present until the continually increasing luminosity of the sun will make the Earth lose all its water or ultimately when the phases that will transform the sun into a red giant star end all life forms on this planet.
It is possible that life did indeed originate in the Noachian eon and continues to exist in certain locations on the planet; however, this is not relevant to the principal discussion here.
These situations do posses realism. If life e.g. emerged and was established at a hydrothermal vent, then this could have exemplified a habitat with a virtually endless energy source but with limitations in space; the organisms adapt to the parameters applicable close to the source and not to the environmental conditions further away. Thus, the organisms can continue to gain thermal energy but compel each other to move outward as they divide. Those pushed outward to a certain distance will not survive, and the hydrothermal vent where this unique population of life on an entire world exists may eventually cease to exist, as environments are dynamic and continue to change. Thus, organisms must be able to leave this environment to initiate biological evolution, because it is risky to merely stay near such a crack in the ocean floor. Of course, a considerable number of life forms exist today both at hydrothermal vents as well as in their surroundings. However, this holds true for biological evolution that has been secured; life today has secured itself with several habitats to support each other. However, in the past scenario discussed, there existed only one habitat where the organisms could live, and there existed only one population of life.
References
Beech M, Coulson IM, Comte M (2018) Lithopanspermia – the terrestrial input during the past 550 million years. American J Astronomy and Astrophys 6(3):81–90
Brandenburg, John E. (1987)"The Paleo-Ocean of Mars". MECA Symposium on Mars: Evolution of its Climate and Atmosphere. Lunar and Planetary Institute
Darwin, Charles, Letter to Joseph Dalton Hooker, 1871, in Darwin, Francis (ed) (1887), The life and letters of Charles Darwin, including an autobiographical chapter, 3 vols. John Murray London
Davies PCW, Lineweaver CH (2011) Cancer tumors as Metazoa 1.0: Tapping genes of ancient ancestors. Phys Biol 8(1):015001
Delaye, Luis, Becerra, Arturo: Cenancestor, the Last Universal Common Ancestor, 2012, Evolution: Education and Outreach 5(3): 382-388
Eagon RG (1962) Psedomonas natrigens, a marine bacterium with a generation time of less than 10 minutes. J Bacteriol 83(4):736–737
Fastook JL, Head JW (2015) Glaciation in the late Noachian icy highlands: ice accumulation, distribution, flow rates, basal melting and top-down melting rates and patterns. Planet Space Sci 106:82–98
Fitch WM, Upper K (1987) The Phylogeny of tRNA sequences provides evidence for ambiguity reduction in the origin of the genetic code, cold spring harbor symp. Quant Biol 52:759–767
Gaucher EAJT, Kratzer JT, Randall RN (2010) Deep phylogeny—how a tree can help characterize early life on Earth. Cold Spring Harb Perspect Biol 2:a002238–a002238
Gibb BC (2015) The organic solar system. Nature Chem 7:364–365
Gibson B, Wilson DJ, Feil E, Eyre-Walker A (2018) The distribution of bacterial doubling times in the wild. Proc Biol Sci 285(1880):20180789
Gilbert W (1986) The RNA World. Nature 319(6055):618
Gogarten JP, Taiz L (1992) Evolution of proton pumping ATPases: Rooting the tree of life. Photosynth Res 33:137–146
Higgs PG (2017) Chemical evolution and the evolutionary definition of life. J Mol Evol 84:225–235
Kim KM, Caetano-Anollés G (2011) The proteomic complexity and rise of the primordial ancestor of diversified life. BMC Evol Biol 11:140
Lehninger, Albert (1982) Principles of Biochemistry, Worth Publishers
Levin PA, Angert ER (2015) Small but mighty: cell size and bacteria. Cold Spring Harb Perspect Biol 7(7):a019216
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20(5):223–228
Michaelian K (2011) Thermodynamic dissipation theory for the origin of life Earth Syst. Dynam 2:37–51
Nicholson WL (2009) Ancient micronauts: interplanetary transport of microbes by cosmic impacts. Trends Microbiol 17(6):243–250
Philippe H, Forterre P (1999) The rooting of the universal tree is not reliable. J Mol Evol 49:509–523
Reid IN, Sparks WB, Lubow S, McGrath M, Lifeio M, Valenti J, Sowers KR, Shukla HD, MacAuley S, Miller T, Suvanasuthi R, Belas R, Colman A, Robb FT, DasSarma P, Müller JA, Coker JA, Cavicchioli R, Chen F, DasSarma S (2006) Terrestrial models for extraterrestrial life: methanogens and halophiles at martian temperatures. Int J Astrobiol 5:89–97
Rich A (1962) On the problems of evolution and biochemical information transfer. In: Kasha M, Pullman B (eds) Horizons in Biochemistry. Academic Press; New York, NY, USA, pp 103–126
Sagan C, Salpeter EE (1976) Particles, environments, and possible ecologies in the Jovian atmosphere. Astrophys J Suppl Series 32:737
Seger J, Brockmann HJ (1987) What is bet-hedging? Oxf Surv Evol Biol 4:182–211
Woese CR, Fox GE (1977) The concept of cellular evolution. J Mol Evol 10:1–6
von Hegner I (2020a) Extremophiles: a special or general case in the search for extra-terrestrial life. Extremophiles 24:167–175
von Hegner I (2020b) Interplanetary transmissions of life in an evolutionary context. Int J Astrobiol 19(4):335–348
von Hegner, Ian (2020c) First principles of terrestrial life: exemplars for potential extra-terrestrial biology, HAL archives-ouvertes.fr CCSD
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
von Hegner, I. The Indeterminacy Bottleneck: Implications for Habitable Worlds. Acta Biotheor 70, 1 (2022). https://doi.org/10.1007/s10441-021-09432-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10441-021-09432-0