Abstract
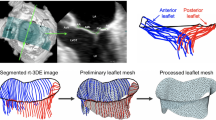
The clinical benefit of patient-specific modeling of heart valve disease remains an unrealized goal, often a result of our limited understanding of the in vivo milieu. This is particularly true in assessing bicuspid aortic valve (BAV) disease, the most common cardiac congenital defect in humans, which leads to premature and severe aortic stenosis or insufficiency (AS/AI). However, assessment of BAV risk for AS/AI on a patient-specific basis is hampered by the substantial degree of anatomic and functional variations that remain largely unknown. The present study was undertaken to utilize a noninvasive computational pipeline (https://doi.org/10.1002/cnm.3142) that directly yields local heart valve leaflet deformation information using patient-specific real-time three-dimensional echocardiographic imaging (rt-3DE) data. Imaging data was collected for patients with normal tricuspid aortic valve (TAV, \(n = 8\)) and those with BAV (\(n = 5\) with fused left and right coronary leaflets and \(n = 5\) with fused right and non-coronary leaflets), from which the medial surface of each leaflet was extracted. The resulting deformation analysis resulted in, for the first time, quantified differences between the in vivo functional deformations of the TAV and BAV leaflets. Our approach was able to capture the complex, heterogeneous surface deformation fields in both TAV and BAV leaflets. We were able to identify and quantify differences in stretch patterns between leaflet types, and found in particular that stretches experienced by BAV leaflets during closure differ from those of TAV leaflets in terms of both heterogeneity as well as overall magnitude. Deformation is a key parameter in the clinical assessment of valvular function, and serves as a direct means to determine regional variations in structure and function. This study is an essential step toward patient-specific assessment of BAV based on correlating leaflet deformation and AS/AI progression, as it provides a means for assessing patient-specific stretch patterns








Similar content being viewed by others
References
Aggarwal, A., G. Ferrari, E. Joyce, M. J. Daniels, R. Sainger, J. H. Gorman, R. Gorman, and M. S. Sacks. Architectural trends in the human normal and bicuspid aortic valve leaflet and its relevance to valve disease. Ann. Biomed. Eng. 42(5):986–998, 2014.
Aggarwal, A., A. M. Pouch, E. Lai, J. Lesicko, P. A. Yushkevich, J. H. Gorman, R. C. Gorman, and M. S. Sacks. In-vivo heterogeneous functional and residual strains in human aortic valve leaflets. J. Biomech. 49(12):2481–2490, 2016.
Ayoub, S., G. Ferrari, R. C. Gorman, J. H. Gorman, F. J. Schoen, and M. S. Sacks. Heart valve biomechanics and underlying mechanobiology. Compr. Physiol. 6(4):1743–1780, 2016.
Ayoub, S., C.-H. Lee, K. H. Driesbaugh, W. Anselmo, C. T. Hughes, G. Ferrari, R. C. Gorman, J. H. Gorman, and M. S. Sacks. Regulation of valve interstitial cell homeostasis by mechanical deformation: implications for heart valve disease and surgical repair. J. R. Soc. Interface. 14(135):20170580, 2017.
Balachandran, K., P. Sucosky, H. Jo, and A. P. Yoganathan. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. Am. J. Physiol.-Heart Circ. Physiol. 296(3):H756–H764, 2009.
Ben Zekry, S., J. Freeman, A. Jajoo, J. He, S. H. Little, G. M. Lawrie, R. Azencott, and W. A. Zoghbi. Patient-specific quantitation of mitral valve strain by computer analysis of three-dimensional echocardiography. Circulation. 9(1):e003254, 2016.
Ben Zekry, S., G. Lawrie, S. Little, W. Zoghbi, J. Freeman, A. Jajoo, S. Jain, J. He, A. Martynenko, and R. Azencott. Comparative evaluation of mitral valve strain by deformation tracking in 3D-echocardiography. Cardiovasc. Eng. Technol. 3(4):402–412, 2012.
Chandra, S., N. M. Rajamannan, and P. Sucosky. Computational assessment of bicuspid aortic valve wall-shear stress: implications for calcific aortic valve disease. Biomech. Model. Mechanobiol. 11(7):1085–1096, 2012.
Davies, M., T. Treasure, and D. Parker. Demographic characteristics of patients undergoing aortic valve replacement for stenosis: relation to valve morphology. Heart. 75(2):174–178, 1996.
Emanuel, R., R. Withers, K. O’Brien, P. Ross, and O. Feizi. Congenitally bicuspid aortic valves clinicogenetic study of 41 families. Heart. 40(12):1402–1407, 1978.
Evangelista, A. Bicuspid aortic valve and aortic root disease. Curr. Cardiol. Rep. 13(3):234–241, 2011.
Huntington, K., A. G. Hunter, and K.-L. Chan. A prospective study to assess the frequency of familial clustering of congenital bicuspid aortic valve. J. Am. Coll. Cardiol. 30(7):1809–1812, 1997.
Khang, A., R. M. Buchanan, S. Ayoub, B. V. Rego, C.-H. Lee, G. Ferrari, K. S. Anseth, and M. S. Sacks. Mechanobiology of the heart valve interstitial cell: Simulation, experiment, and discovery. In: Mechanobiology in Health and Disease, edited by S. Verbruggen. Amsterdam: Elsevier, 2018, pp. 249–283.
Khang, A., R. M. Buchanan, S. Ayoub, B. V. Rego, C.-H. Lee, and M. S. Sacks. Biological mechanics of the heart valve interstitial cell. In: Advances in Heart Valve Biomechanics, edited by M. S. Sacks, and J. Liao. Cham: Springer, 2018, pp. 3–36.
Loop, C. Smooth subdivision surfaces based on triangles. Master’s thesis, University of Utah, 1987.
Merritt, B. A., A. Turin, M. Markl, S. C. Malaisrie, P. M. McCarthy, and J. C. Carr. Association between leaflet fusion pattern and thoracic aorta morphology in patients with bicuspid aortic valve. J. Magn. Reson. Imaging. 40(2):294–300, 2014.
Merryman, W. D., H. D. Lukoff, R. A. Long, G. C. Engelmayr Jr., R. A. Hopkins, and M. S. Sacks. Synergistic effects of cyclic tension and transforming growth factor-β1 on the aortic valve myofibroblast. Cardiovasc. Pathol. 16(5):268–276, 2007.
Narang, H., B. V. Rego, A. H. Khalighi, A. Aly, A. M. Pouch, R. C. Gorman, J. H. Gorman, and M. S. Sacks. Pre-surgical prediction of ischemic mitral regurgitation recurrence using in vivo mitral valve leaflet strains. Ann. Biomed. Eng. 2021. https://doi.org/10.1007/s10439-021-02772-5.
Piegl, L., and W. Tiller. The NURBS Book. New York: Springer, 2012.
Pouch, A. M., S. Tian, M. Takebe, J. Yuan, R. Gorman Jr., A. T. Cheung, H. Wang, B. M. Jackson, J. H. Gorman III., R. C. Gorman, et al. Medially constrained deformable modelling for segmentation of branching medial structures: application to aortic valve segmentation and morphometry. Med. Image Anal. 26(1):217–231, 2015.
Rego, B. V., A. H. Khalighi, A. Drach, E. K. Lai, A. M. Pouch, R. C. Gorman, J. H. Gorman, and M. S. Sacks. A noninvasive method for the determination of in vivo mitral valve leaflet strains. Int. J. Numer. Methods Biomed. Eng. 34(12):e3142, 2018.
Rego, B. V., and M. S. Sacks. A functionally graded material model for the transmural stress distribution of the aortic valve leaflet. J. Biomech. 54:88–95, 2017.
Rego, B. V., S. M. Wells, C.-H. Lee, and M. S. Sacks. Mitral valve leaflet remodelling during pregnancy: insights into cell-mediated recovery of tissue homeostasis. J. R. Soc. Interface. 13(125):20160709, 2016.
Rego, B. V., S. M. Wells, C.-H. Lee, and M. S. Sacks. Remodelling potential of the mitral heart valve leaflet. In: Advances in Heart Valve Biomechanics, edited by M. S. Sacks, and J. Liao. New York: Springer, 2018, pp. 181–206.
Sidak, Z. Rectangular confidence regions for the means of multivariate normal distributions. J. Am. Stat. Assoc. 62(318):626–633, 1967.
Weinberg, E. J., and M. R. K. Mofrad. A multiscale computational comparison of the bicuspid and tricuspid aortic valves in relation to calcific aortic stenosis. J. Biomech. 41(16):3482–3487, 2008.
Xu, F., S. Morganti, R. Zakerzadeh, D. Kamensky, F. Auricchio, A. Reali, T. J. Hughes, M. S. Sacks, and M.-C. Hsu. A framework for designing patient-specific bioprosthetic heart valves using immersogeometric fluid–structure interaction analysis. Int. J. Numer. Methods Biomed. Eng. 34(4):e2938, 2018.
Yap, C.-H., L. P. Dasi, and A. P. Yoganathan. Dynamic hemodynamic energy loss in normal and stenosed aortic valves. J. Biomech. Eng. 132(2):021005, 2010.
Yushkevich, P. A., J. Piven, H. C. Hazlett, R. G. Smith, S. Ho, J. C. Gee, and G. Gerig. Userguided 3d active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 31(3):1116–1128, 2006.
Yushkevich, P. A., H. Zhang, and J. C. Gee. Continuous medial representation for anatomical structures. IEEE Rrans. Med. Imaging. 25(12):1547–1564, 2006.
Zhang, W., R. Zakerzadeh, W. Zhang, and M. S. Sacks. A material modeling approach for the effective response of planar soft tissues for efficient computational simulations. J. Mech. Behav. Biomed. Mater. 89:168–198, 2019.
Acknowledgments
This material was supported by the National Institutes of Health (Grant Nos. R01-HL119297 and R01-HL073021 to M.S.S. and J.H.G.; Grant No. K01-HL141643 to A.M.P.), the National Science Foundation (Grant No. DGE-1610403 to B.V.R.), and the American Heart Association (Grant No. 18PRE34030258 to B.V.R.). The authors gratefully acknowledge Samuel T. Potter for his help with spline surface fitting
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
The image-based stretch estimation method employed in the present study has been previously validated for valvular tissues against both in vitro and in vivo fiducial marker-based deformation metrics.21 However, previous validation studies were performed only for the mitral valve, whose geometry is substantially distinct from that of the AV. To further validate our approach for the specific application to the AV, we applied the stretch estimation method to a computationally generated spline surface geometry of a bioprosthetic TAV implant.27,31 While the bioprosthetic AV has an idealized geometry by design, this validation approach had the advantage of allowing for the comparison of our estimated stretch fields to ground-truth local stretch maps for the modeled TAV, which were obtained via isogeometric analysis.31 Resulting stretches from this validation study showed that our technique was able to accurately capture the complex, heterogeneous leaflet deformation field of the AV (Fig. 9). Specifically, our noninvasive method was able to yield stretch estimates within 5% of their ground-truth value over the entire leaflet surface.
Rights and permissions
About this article
Cite this article
Rego, B.V., Pouch, A.M., Gorman, J.H. et al. Patient-Specific Quantification of Normal and Bicuspid Aortic Valve Leaflet Deformations from Clinically Derived Images. Ann Biomed Eng 50, 1–15 (2022). https://doi.org/10.1007/s10439-021-02882-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02882-0