Abstract
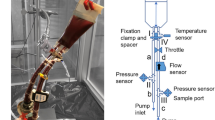
Although the hemocompatibility of left-ventricular assist devices (LVADs) has continuously improved, assessment of hemolysis remains mandatory in pre-clinical testing. The ASTM-F1841 has standardized this assessment since 1997. However, the recommended usage of fresh, non-pooled human blood is hardly feasible with the test loop volume specified therein, when testing the device under test versus a predicate device as required by the international standard 10993-4. In this study, we compared ASTM-conforming (ASTM) and downscaled (mini) test loops with a one-third priming volume for the assessment of blood damage at the ASTM operating point. Blood damage was assessed for HeartMate 3 and BPX-80 in 6 experiments with heparinized porcine slaughterhouse blood for 6 h. We analyzed plasma free hemoglobin (pfHb), von Willebrand factor (vWF) concentration and collagen-binding functionality and calculated indices of hemolysis and vWF-ratios. The mini test loops provided significantly higher pfHb increase and consistently stronger vWF-ratio decrease and yielded a significantly better differentiation of the pumps. Interestingly, indices of hemolysis were generally lower in the mini set-up, indicating less adverse effects by the mini loop itself. Thus, we propose our mini test loop as suitable tool for clinically relevant standardized assessment of blood damage by future LVADs with single-donation human blood.





Similar content being viewed by others
References
Adachi, T., T. Matsushita, Z. Dong, A. Katsumi, T. Nakayama, T. Kojima, H. Saito, J. E. Sadler, and T. Naoe. Identification of amino acid residues essential for heparin binding by the A1 domain of human von Willebrand factor. Biochem. Biophys. Res. Commun. 339:1178–1183, 2006. https://doi.org/10.1016/j.bbrc.2005.11.126.
ASTM International. ASTM F1841-97. Standard Practice for Assessment of Hemolysis in Continuous Flow blood Pumps. West Conshohocken, PA: ASTM International, 2017. https://doi.org/10.1520/f1841-97r17.
Bourque, K., C. Cotter, C. Dague, D. Harjes, O. Dur, J. Duhamel, K. Spink, K. Walsh, and E. Burke. Design rationale and preclinical evaluation of the HeartMate 3 left ventricular assist system for hemocompatibility. ASAIO J. 62:375–383, 2016. https://doi.org/10.1097/MAT.0000000000000388.
Bryckaert, M., J.-P. Rosa, C. V. Denis, and P. J. Lenting. Of von Willebrand factor and platelets. Cell. Mol. Life Sci. 72:307–326, 2015. https://doi.org/10.1007/s00018-014-1743-8.
Coghill, P. A., S. Kanchi, Z. J. Azartash-Namin, J. W. Long, and T. A. Snyder. Benchtop von Willebrand factor testing: comparison of commercially available ventricular assist devices and evaluation of variables for a standardized test method. ASAIO J. 2018. https://doi.org/10.1097/MAT.0000000000000849.
Crow, S., D. Chen, C. Milano, W. Thomas, L. Joyce, V. Piacentino, R. Sharma, J. Wu, G. Arepally, D. Bowles, J. Rogers, and N. Villamizar-Ortiz. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann. Thorac. Surg. 90:1263–1269, 2010. https://doi.org/10.1016/j.athoracsur.2010.04.099.
Da, Q., M. Teruya, P. Guchhait, J. Teruya, J. S. Olson, and M. A. Cruz. Free hemoglobin increases von Willebrand factor-mediated platelet adhesion in vitro: implications for circulatory devices. Blood 126:2338–2341, 2015. https://doi.org/10.1182/blood-2015-05-648030.
DIN 58931:2010-08. Haematology—Determination of Haemoglobin Concentration in Blood—Reference Method. Berlin: Beuth Verlag GmbH, 2010. https://doi.org/10.31030/1623224.
DIN EN ISO 10993-4:2017-12. Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood (ISO 10993-4:2017); German Version EN_ISO_10993-4:2017. Berlin: Beuth Verlag GmbH, 2017. https://doi.org/10.31030/2597308.
Egger, C., J. Maas, T. Hufen, T. Schmitz-Rode, and U. Steinseifer. Establishing a method for in vitro investigation of mechanical parameters causing acquired von Willebrand syndrome in ventricular assist devices. Artif. Organs 37:833–839, 2013. https://doi.org/10.1111/aor.12116.
Farrar, D. J., K. Bourque, C. P. Dague, C. J. Cotter, and V. L. Poirier. Design features, developmental status, and experimental results with the Heartmate III centrifugal left ventricular assist system with a magnetically levitated rotor. ASAIO J. 53:310–315, 2007. https://doi.org/10.1097/MAT.0b013e3180536694.
Gao, C., B. Boylan, J. Fang, D. A. Wilcox, D. K. Newman, and P. J. Newman. Heparin promotes platelet responsiveness by potentiating αIIbβ3-mediated outside-in signaling. Blood 117:4946–4952, 2011. https://doi.org/10.1182/blood-2010-09-307751.
Geisen, U., K. Brehm, G. Trummer, M. Berchtold-Herz, C. Heilmann, F. Beyersdorf, J. Schelling, A. Schlagenhauf, and B. Zieger. Platelet secretion defects and acquired von Willebrand syndrome in patients with ventricular Assist devices. J. Am. Heart. Assoc. 2018. https://doi.org/10.1161/jaha.117.006519.
Hijikata, W., T. Shinshi, J. Asama, L. Li, H. Hoshi, S. Takatani, and A. Shimokohbe. A magnetically levitated centrifugal blood pump with a simple-structured disposable pump head. Artif. Organs 32:531–540, 2008. https://doi.org/10.1111/j.1525-1594.2008.00576.x.
Hoshi, H., J. Asama, W. Hijikata, C. Hara, T. Shinshi, T. Yasuda, K. Ohuchi, A. Shimokohbe, and S. Takatani. Hemolytic performance of a MagLev disposable rotary blood pump (MedTech Dispo): effects of MagLev gap clearance and surface roughness. Artif. Organs 30:949–954, 2006. https://doi.org/10.1111/j.1525-1594.2006.00332.x.
Kormos, R. L., J. Cowger, F. D. Pagani, J. J. Teuteberg, D. J. Goldstein, J. P. Jacobs, R. S. Higgins, L. W. Stevenson, J. Stehlik, P. Atluri, K. L. Grady, and J. K. Kirklin. The Society of Thoracic Surgeons Intermacs database annual report: evolving indications, outcomes, and scientific partnerships. J. Heart Lung Transplant. 38:114–126, 2019. https://doi.org/10.1016/j.healun.2018.11.013.
Mehra, M. R., D. J. Goldstein, N. Uriel, J. C. Cleveland, M. Yuzefpolskaya, C. Salerno, M. N. Walsh, C. A. Milano, C. B. Patel, G. A. Ewald, A. Itoh, D. Dean, A. Krishnamoorthy, W. G. Cotts, A. J. Tatooles, U. P. Jorde, B. A. Bruckner, J. D. Estep, V. Jeevanandam, G. Sayer, D. Horstmanshof, J. W. Long, S. Gulati, E. R. Skipper, J. B. O’Connell, G. Heatley, P. Sood, and Y. Naka. Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N. Engl. J. Med. 378:1386–1395, 2018. https://doi.org/10.1056/NEJMoa1800866.
Meyer, A. L., D. Malehsa, C. Bara, U. Budde, M. S. Slaughter, A. Haverich, and M. Strueber. Acquired von Willebrand syndrome in patients with an axial flow left ventricular assist device. Circ. Heart Fail. 3:675–681, 2010. https://doi.org/10.1161/CIRCHEARTFAILURE.109.877597.
Meyer, A. L., D. Malehsa, U. Budde, C. Bara, A. Haverich, and M. Strueber. Acquired von Willebrand syndrome in patients with a centrifugal or axial continuous flow left ventricular assist device. JACC 2:141–145, 2014. https://doi.org/10.1016/j.jchf.2013.10.008.
Moazami, N., K. Fukamachi, M. Kobayashi, N. G. Smedira, K. J. Hoercher, A. Massiello, S. Lee, D. J. Horvath, and R. C. Starling. Axial and centrifugal continuous-flow rotary pumps: a translation from pump mechanics to clinical practice. J. Heart Lung Transplant. 32:1–11, 2013. https://doi.org/10.1016/j.healun.2012.10.001.
Motulsky, H. J., and R. E. Brown. Detecting outliers when fitting data with nonlinear regression—a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 7:123, 2006. https://doi.org/10.1186/1471-2105-7-123.
Mueller, M. R., H. Schima, H. Engelhardt, A. Salat, D. B. Olsen, U. Losert, and E. Wolner. In vitro hematological testing of rotary blood pumps: remarks on standardization and data interpretation. Artif. Organs 17:103–110, 1993. https://doi.org/10.1111/j.1525-1594.1993.tb00419.x.
Müller, J. P., A. Löf, S. Mielke, T. Obser, L. K. Bruetzel, W. Vanderlinden, J. Lipfert, R. Schneppenheim, and M. Benoit. pH-Dependent Interactions in dimers govern the mechanics and structure of von Willebrand factor. Biophys. J. 111:312–322, 2016. https://doi.org/10.1016/j.bpj.2016.06.022.
Netuka, I., T. Kvasnička, J. Kvasnička, I. Hrachovinová, P. Ivák, F. Mareček, J. Bílková, I. Malíková, M. Jančová, J. Maly, P. Sood, K. S. Sundareswaran, J. M. Connors, and M. R. Mehra. Evaluation of von Willebrand factor with a fully magnetically levitated centrifugal continuous-flow left ventricular assist device in advanced heart failure. J. Heart Lung Transplant. 35:860–867, 2016. https://doi.org/10.1016/j.healun.2016.05.019.
Olia, S. E., T. M. Maul, J. F. Antaki, and M. V. Kameneva. Mechanical blood trauma in assisted circulation: sublethal RBC damage preceding hemolysis. Int. J. Artif. Organs 39:150–159, 2016. https://doi.org/10.5301/ijao.5000478.
Restle, D. J., D. M. Zhang, G. Hung, J. L. Howard, F. Kallel, M. A. Acker, P. Atluri, and C. R. Bartoli. Preclinical models for translational investigations of left ventricular assist device-associated von Willebrand factor degradation. Artif. Organs 39:569–575, 2015. https://doi.org/10.1111/aor.12428.
Rosenberg, G., C. A. Siedlecki, C.-S. Jhun, W. J. Weiss, K. Manning, S. Deutsch, and W. Pierce. Acquired von Willebrand syndrome and blood pump design. Artif. Organs 42:1119–1124, 2018. https://doi.org/10.1111/aor.13291.
Sakota, D., R. Sakamoto, H. Sobajima, N. Yokoyama, S. Waguri, K. Ohuchi, and S. Takatani. Mechanical damage of red blood cells by rotary blood pumps: selective destruction of aged red blood cells and subhemolytic trauma. Artif. Organs 32:785–791, 2008. https://doi.org/10.1111/j.1525-1594.2008.00631.x.
Sobel, M., P. M. McNeill, P. L. Carlson, J. C. Kermode, B. Adelman, R. Conroy, and D. Marques. Heparin inhibition of von Willebrand factor-dependent platelet function in vitro and in vivo. J. Clin. Invest. 87:1787–1793, 1991. https://doi.org/10.1172/JCI115198.
Susen, S., A. Rauch, E. van Belle, A. Vincentelli, and P. J. Lenting. Circulatory support devices: fundamental aspects and clinical management of bleeding and thrombosis. J. Thromb. Haemost. 13:1757–1767, 2015. https://doi.org/10.1111/jth.13120.
Tiede, A., J. Priesack, S. Werwitzke, K. Bohlmann, B. Oortwijn, P. Lenting, R. Eisert, A. Ganser, and U. Budde. Diagnostic workup of patients with acquired von Willebrand syndrome: a retrospective single-centre cohort study. J. Thromb. Haemost. 6:569–576, 2008. https://doi.org/10.1111/j.1538-7836.2008.02909.x.
Xu, A. J., and T. A. Springer. Calcium stabilizes the von Willebrand factor A2 domain by promoting refolding. Proc. Natl. Acad. Sci. USA. 109:3742–3747, 2012. https://doi.org/10.1073/pnas.1121261109.
Zayat, R., A. Moza, O. Grottke, T. Grzanna, T. Fechter, T. Motomura, C. Schmidt-Mewes, T. Breuer, R. Autschbach, R. Rossaint, A. Goetzenich, and C. Bleilevens. In vitro comparison of the hemocompatibility of two centrifugal left ventricular assist devices. J. Thorac. Cardiovasc. Surg. 157:591–599.e4, 2019. https://doi.org/10.1016/j.jtcvs.2018.07.085.
Acknowledgments
The authors thank Thomas Berg from the Department of Thoracic and Cardiovascular Surgery, Medical Faculty, RWTH Aachen University, Aachen, Germany, for the loan of the HM3 and equipment. This study was supported by the Elisabeth and Rudolf Hirsch Foundation for Medical Research (Cologne, Germany) and the European Regional Development Fund (ERDF) of the European Union and North-Rhine Westphalia (Grant Number: EFRE-0800410).
Conflict of interest
The authors declare no conflicts of interest regarding this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ender A Finol oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Woelke, E., Klein, M., Mager, I. et al. Miniaturized Test Loop for the Assessment of Blood Damage by Continuous-Flow Left-Ventricular Assist Devices. Ann Biomed Eng 48, 768–779 (2020). https://doi.org/10.1007/s10439-019-02404-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02404-z