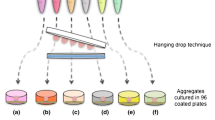
Abstract
Given the low self-healing capacity of fibrocartilage and hyaline cartilage, tissue engineering holds great promise for the development of new regenerative therapies. However, dedifferentiation of cartilage cells during expansion leads to fibrous tissue instead of cartilage. The purpose of our study was to generate 3D microtissues, spheroids, mimicking the characteristics of native fibrocartilage or articular cartilage to use as modular units for implantation in meniscal and articular cartilage lesions, respectively, within the knee joint. A set of parameters was assessed to create spheroids with a geometry compatible with 3D bioprinting for the creation of a biomimetic cartilage construct. Fibrochondrocytes (FC) and articular chondrocytes (AC) spheroids were created using a high-throughput microwell system. Spheroid morphology, viability, proliferation and extracellular matrix were extensively screened. After 2D expansion, FC and AC dedifferentiated, resulting in a loss of cartilage specific extracellular matrix proteins. Spheroid formation did not result in FC redifferentiation, but did lead to redifferentiation of AC, resulting in microtissues displaying collagen II, aggrecan and glycosaminoglycans. This study demonstrates 3D cartilage mimics that could have a potential application in the next generation of Autologous Chondrocyte Implantation procedures. Moreover, spheroids can be used as building blocks to create cartilage constructs by bioprinting in the future.







Similar content being viewed by others
References
Adesida, A. B., L. M. Grady, W. S. Khan, S. J. Millward-Sadler, D. M. Salter, and T. E. Hardingham. Human meniscus cells express hypoxia inducible factor-1alpha and increased SOX9 in response to low oxygen tension in cell aggregate culture. Arthritis Res. Ther. 9:R69, 2007.
Anada, T., C.-C. Pan, A. Stahl, S. Mori, J. Fukuda, O. Suzuki, and Y. Yang. Vascularized bone-mimetic hydrogel constructs by 3D bioprinting to promote osteogenesis and angiogenesis. Int. J. Mol. Sci. 20:1096, 2019.
Anderer, U., and J. Libera. In vitro engineering of human autogenous cartilage. J. Bone Miner. Res. 17:1420–1429, 2002.
Anderson, D. E. J., and K. A. Athanasiou. Passaged goat costal chondrocytes provide a feasible cell source for temporomandibular joint tissue engineering. Ann. Biomed. Eng. 36:1992–2001, 2008.
Babur, B. K., et al. The interplay between chondrocyte redifferentiation pellet size and oxygen concentration. PLoS ONE 8:e58865, 2013.
Basad, E., F. R. Wissing, P. Fehrenbach, M. Rickert, J. Steinmeyer, and B. Ishaque. Matrix-induced autologous chondrocyte implantation (MACI) in the knee: clinical outcomes and challenges. Knee Surg. Sport Traumatol. Arthrosc. 23:3729–3735, 2015.
Becher, C., V. Laute, S. Fickert, W. Zinser, P. Niemeyer, T. John, P. Diehl, T. Kolombe, R. Siebold, and J. Fay. Safety of three different product doses in autologous chondrocyte implantation: results of a prospective, randomised, controlled trial. J. Orthop. Surg. Res. 12:71, 2017.
Berneel, E., C. Philips, H. Declercq, and R. Cornelissen. Redifferentiation of high-throughput generated fibrochondrocyte micro-aggregates: impact of low oxygen tension. Cells Tissues Organs 202:369–381, 2016.
Bhosale, A. M., and J. B. Richardson. Articular cartilage: structure, injuries and review of management. Br. Med. Bull. 87:77–95, 2008.
Billiet, T., E. Gevaert, T. De Schryver, M. Cornelissen, and P. Dubruel. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 35:49–62, 2014.
Caron, M. M. J., P. J. Emans, M. M. E. Coolsen, L. Voss, D. A. M. Surtel, A. Cremers, L. W. van Rhijn, and T. J. M. Welting. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthr. Cartil. 20:1170–1178, 2012.
Chu, C. R., M. Szczodry, and S. Bruno. Animal models for cartilage regeneration and repair. Tissue Eng. Part B Rev. 16:105–115, 2010.
Cui, X., A. Hasegawa, M. Lotz, and D. D’Lima. Structured three-dimensional co-culture of mesenchymal stem cells with meniscus cells promotes meniscal phenotype without hypertrophy. Biotechnol. Bioeng. 109:2369–2380, 2012.
Daly, A. C., et al. A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 8:045002, 2016.
Darling, E. M., and K. A. Athanasiou. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 23:425–432, 2005.
De Moor, L., I. Merovci, S. Baetens, J. Verstraeten, P. Kowalska, D. V. Krysko, W. H. De Vos, and H. Declercq. High-throughput fabrication of vascularized spheroids for bioprinting. Biofabrication 10(3):35009, 2018.
Deponti, D., A. Di Giancamillo, C. Scotti, G. M. Peretti, and I. Martin. Animal models for meniscus repair and regeneration. J. Tissue Eng. Regen. Med. 9:512–527, 2015.
Duval, K., H. Grover, L. H. Han, Y. Mou, A. F. Pegoraro, J. Fredberg, and Z. Chen. Modeling physiological events in 2D vs. 3D cell culture. Physiology 32:266–277, 2017.
Feng, J., K. Mineda, S.-H. Wu, T. Mashiko, K. Doi, S. Kuno, K. Kinoshita, K. Kanayama, R. Asahi, A. Sunaga, and K. Yoshimura. An injectable non-cross-linked hyaluronic-acid gel containing therapeutic spheroids of human adipose-derived stem cells. Sci. Rep. 7:1548, 2017.
Fox, A. J. S., F. Wanivenhaus, A. J. Burge, R. F. Warren, and S. A. Rodeo. The human meniscus: a review of anatomy, function, injury, and advances in treatment. Clin. Anat. 28:269–287, 2015.
Freymann, U., M. Endres, U. Goldmann, M. Sittinger, and C. Kaps. Toward scaffold-based meniscus repair: effect of human serum, hyaluronic acid and TGF-ß3 on cell recruitment and re-differentiation. Osteoarthr. Cartil. 21:773–781, 2013.
Futrega, K., J. S. Palmer, M. Kinney, W. B. Lott, M. D. Ungrin, P. W. Zandstra, and M. R. Doran. The microwell-mesh: A novel device and protocol for the high throughput manufacturing of cartilage microtissues. Biomaterials 62:1–12, 2015.
Grogan, S. P., C. Pauli, M. K. Lotz, and D. D. D’Lima. Relevance of meniscal cell regional phenotype to tissue engineering. Connect. Tissue Res. 58:259–270, 2017.
Gunja, N. J., and K. A. Athanasiou. Passage and reversal effects on gene expression of bovine meniscal fibrochondrocytes. Arthritis Res. Ther. 9:R93, 2007.
Hoben, G. M., J. C. Hu, R. A. James, and K. A. Athanasiou. Self-assembly of fibrochondrocytes and chondrocytes for tissue engineering of the knee meniscus. Tissue Eng. 13:939–946, 2007.
Huang, B. J., J. C. Hu, and K. A. Athanasiou. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 98:1–22, 2016.
Ibold, Y., S. Frauenschuh, C. Kaps, M. Sittinger, J. Ringe, and P. M. Goetz. Development of a high-throughput screening assay based on the 3-dimensional pannus model for rheumatoid arthritis. J. Biomol. Screen. 12:956–965, 2007.
Korpershoek, J. V., T. S. de Windt, M. H. Hagmeijer, L. A. Vonk, and D. B. Saris. Cell-based meniscus repair and regeneration: at the brink of clinical translation? A systematic review of preclinical studies. Orthopaedic Journal of Sports Medicine. 5(2):2325967117690131, 2017.
Krill, M., N. Early, J. S. Everhart, and D. C. Flanigan. Autologous chondrocyte implantation (ACI) for knee cartilage defects. JBJS Rev. 6:e5, 2018.
Madeira, C., A. Santhagunam, J. B. Salgueiro, and J. M. S. Cabral. Advanced cell therapies for articular cartilage regeneration. Trends Biotechnol. 33:35–42, 2015.
Markway, B. D., G.-K. Tan, G. Brooke, J. E. Hudson, J. J. Cooper-White, and M. R. Doran. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant. 19:29–42, 2010.
Marsano, A., S. J. Millward-Sadler, D. M. Salter, A. Adesida, T. Hardingham, E. Tognana, E. Kon, C. Chiari-Grisar, S. Nehrer, M. Jakob, and I. Martin. Differential cartilaginous tissue formation by human syno vial membrane, fat pad, meniscus cells and articular chondrocytes. Osteoarthr. Cartil. 15:48–58, 2007.
Martinez, I., J. Elvenes, R. Olsen, K. Bertheussen, and O. Johansen. Redifferentiation of in vitro expanded adult articular chondrocytes by combining the hanging-drop cultivation method with hypoxic environment. Cell Transplant. 17:987–996, 2008.
McCarthy, H. S., and S. Roberts. A histological comparison of the repair tissue formed when using either Chondrogide® or periosteum during autologous chondrocyte implantation. Osteoarthr. Cartil. 21:2048–2057, 2013.
Medvedeva, E., E. Grebenik, S. Gornostaeva, V. Telpuhov, A. Lychagin, P. Timashev, and A. Chagin. Repair of damaged articular cartilage: current approaches and future directions. Int. J. Mol. Sci. 19(8):2366, 2018.
Mironov, V., R. P. Visconti, V. Kasyanov, G. Forgacs, C. J. Drake, and R. R. Markwald. Organ printing: tissue spheroids as building blocks. Biomaterials 30:2164–2174, 2009.
Moldovan, N., L. Maldovan, and M. Raghunath. Of balls, inks and cages: Hybrid biofabrication of 3D tissue analogs. Int. J. Bioprinting 5:1, 2018.
Mouser, V. H. M., R. Levato, L. J. Bonassar, D. D. D’Lima, D. A. Grande, T. J. Klein, D. B. F. Saris, M. Zenobi-Wong, D. Gawlitta, and J. Malda. Three-dimensional bioprinting and its potential in the field of articular cartilage regeneration. Cartilage 8:327–340, 2017.
Murphy, M. K., T. E. Masters, J. C. Hu, and K. A. Athanasiou. Engineering a fibrocartilage spectrum through modulation of aggregate redifferentiation. Cell Transplant. 24:235–245, 2015.
Negoro, T., Y. Takagaki, H. Okura, and A. Matsuyama. Trends in clinical trials for articular cartilage repair by cell therapy. NPJ Regen. Med. 3:17, 2018.
Nichol, J. W., and A. Khademhosseini. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter 5:1312–1319, 2009.
Ovsianikov, A., A. Khademhosseini, and V. Mironov. The synergy of scaffold-based and scaffold-free tissue engineering strategies. Trends Biotechnol. 36:348–357, 2018.
Peck, Y., L. T. Leom, P. F. P. Low, and D.-A. Wang. Establishment of an in vitro three-dimensional model for cartilage damage in rheumatoid arthritis. J. Tissue Eng. Regen. Med. 12:e237–e249, 2018.
Penick, K. J., L. A. Solchaga, and J. F. Welter. High-throughput aggregate culture system to assess the chondrogenic potential of mesenchymal stem cells. Biotechniques 39:687–691, 2005.
Rezende, R. A., F. D. A. S. Pereira, V. Kasyanov, D. T. Kemmoku, I. Maia, J. V. L. da Silva, and V. Mironov. Scalable biofabrication of tissue spheroids for organ printing. Proc. CIRP 5:276–281, 2013.
Ruedel, A., S. Hofmeister, and A.-K. Bosserhoff. Development of a model system to analyze chondrogenic differentiation of mesenchymal stem cells. Int. J. Clin. Exp. Pathol. 6:3042–3048, 2013.
Schon, B. S., G. J. Hooper, and T. B. F. Woodfield. Modular tissue assembly strategies for biofabrication of engineered cartilage. Ann. Biomed. Eng. 45:100–114, 2017.
Schubert, T., S. Anders, E. Neumann, J. Schölmerich, F. Hofstädter, J. Grifka, U. Müller-Ladner, J. Libera, and J. Schedel. Long-term effects of chondrospheres on cartilage lesions in an autologous chondrocyte implantation model as investigated in the SCID mouse model. Int. J. Mol. Med. 23:455–460, 2009.
Smith-Mungo, L. I., and H. M. Kagan. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 16:387–398, 1998.
Sun, L., X. Wang, and D. L. Kaplan. A 3D cartilage—inflammatory cell culture system for the modeling of human osteoarthritis. Biomaterials 32:5581–5589, 2011.
Tan, G.-K., D. L. M. Dinnes, P. T. Myers, and J. J. Cooper-White. Effects of biomimetic surfaces and oxygen tension on redifferentiation of passaged human fibrochondrocytes in 2D and 3D cultures. Biomaterials 32:5600–5614, 2011.
Tarafder, S., J. Gulko, D. Kim, K. H. Sim, S. Gutman, J. Yang, J. L. Cook, and C. H. Lee. Effect of dose and release rate of CTGF and TGFβ3 on avascular meniscus healing. J. Orthop. Res. 2019. https://doi.org/10.1002/jor.24287.
Teixeira, L. M., J. C. Leijten, J. Sobral, R. Jin, A. A. van Apeldoorn, J. Feijen, C. van Blitterswijk, P. J. Dijkstra, and M. Karperien. High throughput generated micro-aggregates of chondrocytes stimulate cartilage formation in vitro and in vivo. Eur. Cell Mater. 23:387–399, 2012.
Upton, M. L., J. Chen, and L. A. Setton. Region-specific constitutive gene expression in the adult porcine meniscus. J. Orthop. Res. 24:1562–1570, 2006.
Ushiki, T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch. Histol. Cytol. 65:109–126, 2002.
Welter, J. F., L. A. Solchaga, and K. J. Penick. Simplification of aggregate culture of human mesenchymal stem cells as a chondrogenic screening assay. Biotechniques 42:732–737, 2007.
Young, B., J. Lowe, A. Stevens, and J. Heath. Wheater’s functional histology. A text and colour Atlas. Amsterdam: Elsevier, 2006.
Yu, Y., K. K. Moncal, J. Li, W. Peng, I. Rivero, J. A. Martin, and I. T. Ozbolat. Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink. Sci. Rep. 6:28714, 2016.
Zhang, L., P. Su, C. Xu, J. Yang, W. Yu, and D. Huang. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol. Lett. 32:1339–1346, 2010.
Acknowledgments
All authors declare no conflicts of interest and disclose any financial and personal relationships with persons or organizations that could potentially bias this work and conclusions. The authors would like to thank ORSI Melle for providing the biological materials, Prof. Dr. Jan Vanfleteren and NaMiFab Ghent University for kindly providing the PDMS molds and Prof. Dr. Björn Heindryckx and Prof. Dr. Jolanda van Hengel (Ghent University) for access to the low oxygen incubator. We also would like to thank Greet De Smet, Leen Pieters and Johanna Aernoudt (Ghent University) for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Kent Leach oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De Moor, L., Beyls, E. & Declercq, H. Scaffold Free Microtissue Formation for Enhanced Cartilage Repair. Ann Biomed Eng 48, 298–311 (2020). https://doi.org/10.1007/s10439-019-02348-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02348-4