Abstract
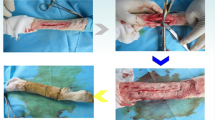
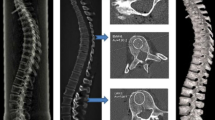
The aim of this study was to investigate the influences of low-magnitude high-frequency vibration (LMHFV) with different rest period regimes (vibrational loading per day [with or without the loading divided into bouts]; or vibrational loading for 7 day followed by 7 day rest [with or without the loading divided into bouts]) on bone healing at multi-levels. Transverse fractures of rat bilateral tibias were established using a Kirschner wire inserted for fixation. The animals were randomly assigned to five groups (n = 7 for each group): four for vibrational groups by LMHFV with different rest period regimes and one for fractured model without mechanical loading. The macromechanical properties of the fractured tibias and the nanomechanical properties of the calluses were investigated through three-point bending and nanoindentation tests, respectively. Atomic force microscopy (AFM) was performed to analyze the nanostructure of the calluses. Micro-computed tomography (micro-CT) scanning was conducted to evaluate the microarchitecture of the calluses. The serum concentration of osteocalcin (OG) and tartrate-resistant acid phosphatase 5b (TRAP5b) were measured to assess the bone formation and resorption rates, respectively. Significantly higher values of failure load and elastic modulus were observed in DL (vibrational loading for 15 min per day) and DLR (vibrational loading per day in which three bouts of 5 min of vibration were separated by 4 h) than FBC (fractured model without mechanical loading) at macro-level (P < 0.05). The results of nanoindentation test showed the highest values of indentation modulus and hardness in DLR (significantly higher than FBC; P < 0.05); besides, higher value of hardness was also observed in DL (significantly higher than FBC; P < 0.05). Though AFM imaging showed no significant differences in grain sizes between the vibrational groups and FBC, roughness of DLR showed the highest value, i.e. it was significantly higher than that in FBC (P < 0.05). For microarchitectural parameters obtained from micro-CT imaging, tissue mineral density (TMD) of DLR and VL7 (vibrational loading for 7 day followed by a 7-day rest, 15 min per day during vibrational periods) were significantly higher than that in FBC (P < 0.05), but no significant differences in other parameters were observed between vibrational groups and FBC. There were no significant differences of OG between vibrational groups and FBC; however, FBC showed significantly higher TRAP5b concentration than all vibrational groups (P < 0.05). The results show that LMHFV with different rest period regimes not only altered the macro- and nano-level bone mechanical properties but also influenced the TMD of calluses and nano-level spatial arrangement (roughness) significantly. The most significant effect of LMHFV with different rest period regimes was observed in DLR, which indicated that both osteogenic accumulation and cellular resensitization can be satisfied under this regimen. Hence, the DLR group demonstrated a great potential in clinical applications.












Similar content being viewed by others
References
Augat, P., K. Margevicius, J. Simon, S. Wolf, G. Suger, and L. Claes. Local tissue properties in bone healing: influence of size and stability of the osteotomy gap. J. Orthop. Res. 16(4):475–481, 1998.
Beck, B. R. Vibration therapy to prevent bone loss and falls: mechanisms and efficacy. Curr. Osteoporos. Rep. 13(6):381–389, 2015.
Bonnarens, F., and T. A. Einhorn. Production of a standard closed fracture in laboratory animal bone. J. Orthop. Res. 2:97–101, 1984.
Charles, P., J. W. Poser, L. Mosekilde, and F. T. Jensen. Estimation of bone turnover evaluated by 47Ca-kinetics. Efficiency of serum bone gamma-carboxyglutamic acid-containing protein, serum alkaline phosphatase, and urinary hydroxyproline excretion. J. Clin. Investig. 76(6):2254–2258, 1985.
Chow, D. H., K. S. Leung, L. Qin, A. H. Leung, and W. H. Cheung. Low-magnitude high-frequency vibration (LMHFV) enhances bone remodeling in osteoporotic rat femoral fracture healing. J. Orthop. Res. 29(5):746–752, 2011.
Gao, J., H. Gong, X. Huang, J. Fang, D. Zhu, and Y. Fan. Relationship between microstructure, material distribution, and mechanical properties of sheep tibia during fracture healing process. Int. J. Med. Sci. 10(11):1560–1569, 2013.
Garman, R., G. Gaudette, L. R. Donahue, C. Rubin, and S. Judex. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J. Orthop. Res. 25(6):732–740, 2007.
Gusi, N., A. Raimundo, and A. Leal. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: a randomized controlled trial. BMC. Musculoskelet. Disord. 7:92, 2006.
Halleen, J. M., S. L. Alatalo, H. Suominen, S. Cheng, A. J. Janckila, and H. K. Vaananen. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J. Bone Miner. Res. 15(7):1337–1345, 2000.
Ishimoto, T., T. Nakano, M. Yamamoto, and Y. Tabata. Biomechanical evaluation of regenerating long bone by nanoindentation. J. Mater. Sci. Mater. Med. 22(4):969–976, 2011.
Judex, S., X. Lei, D. Han, and C. Rubin. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J. Biomech. 40(6):1333–1339, 2007.
Kulkarni, R. N., P. A. Voglewede, and D. Liu. Mechanical vibration inhibits osteoclast formation by reducing DC-STAMP receptor expression in osteoclast precursor cells. Bone 57(2):493–498, 2013.
Lam, H., M. Hu, and Y. X. Qin. Alteration of contraction-to-rest ratio to optimize trabecular bone adaptation induced by dynamic muscle stimulation. Bone. 48(2):399–405, 2011.
Lau, E., S. Al-Dujaili, A. Guenther, D. Liu, L. Wang, and L. You. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone 46(6):1508–1515, 2010.
Leung, K. S., H. F. Shi, W. H. Cheung, L. Qin, W. K. Ng, K. F. Tam, and N. Tang. Low-magnitude high-frequency vibration accelerates callus formation, mineralization, and fracture healing in rats. J. Orthop. Res. 27(4):458–465, 2009.
Liphardt, A. M., J. Schipilow, D. A. Hanley, and S. K. Boyd. Bone quality in osteopenic postmenopausal women is not improved after 12 months of whole-body vibration training. Osteoporos. Int. 26(3):911–920, 2015.
Lynch, M. A., M. D. Brodt, and M. J. Silva. Skeletal effects of whole-body vibration in adult and aged mice. J. Orthop. Res. 28(2):241–247, 2010.
Lynch, M. A., M. D. Brodt, A. L. Stephens, R. Civitelli, and M. J. Silva. Low-magnitude whole-body vibration does not enhance the anabolic skeletal effects of intermittent PTH in adult mice. J. Orthop. Res. 29(4):465–472, 2011.
Ma, R., D. Zhu, H. Gong, G. Gu, X. Huang, J. Gao, and X. Zhang. High-frequency and low-magnitude whole body vibration with rest days is more effective in improving skeletal micro-morphology and biomechanical properties in ovariectomised rodents. Hip Int. 22(2):218–226, 2012.
Manjubala, I., Y. Liu, D. R. Epari, P. Roschger, H. Schell, P. Fratzl, and G. N. Duda. Spatial and temporal variations of mechanical properties and mineral content of the external callus during bone healing. Bone 45(2):185–192, 2009.
Manske, S. L., C. A. Good, R. F. Zernicke, and S. K. Boyd. High-frequency, low-magnitude vibration does not prevent bone loss resulting from muscle disuse in mice following botulinum toxin injection. PLoS One 7:e36486, 2012.
Milovanovic, P., M. Djuric, and Z. Rakocevic. Age-dependence of power spectral density and fractal dimension of bone mineralized matrix in atomic force microscope topography images: potential correlates of bone tissue age and bone fragility in female femoral neck trabeculae. J. Anat. 221(5):427–433, 2012.
Milovanovic, P., J. Potocnik, D. Djonic, S. Nikolic, V. Zivkovic, M. Djuric, and Z. Rakocevic. Age-related deterioration in trabecular bone mechanical properties at material level: nanoindentation study of the femoral neck in women by using AFM. Exp. Gerontol. 47(2):154–159, 2012.
Nyman, J. S., S. Munoz, S. Jadhav, A. Mansour, T. Yoshii, G. R. Mundy, and G. E. Gutierrez. Quantitative measures of femoral fracture repair in rats derived by micro-computed tomography. J. Biomech. 42(7):891–897, 2009.
Robling, A. G., D. B. Burr, and C. H. Turner. Recovery periods restore mechanosensitivity to dynamically loaded bone. J. Exp. Biol. 204(Pt 19):3389–3399, 2001.
Robling, A. G., F. M. Hinant, D. B. Burr, and C. H. Turner. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J. Bone Miner. Res. 17(8):1545–1554, 2002.
Shi, H. F., W. H. Cheung, L. Qin, A. H. Leung, and K. S. Leung. Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone. Bone 46(5):1299–1305, 2010.
Shidara, K., M. Inaba, S. Okuno, S. Yamada, Y. Kumeda, Y. Imanishi, T. Yamakawa, E. Ishimura, and Y. Nishizawa. Serum levels of TRAP5b, a new bone resorption marker unaffected by renal dysfunction, a new bone as a useful marker of cortical bone loss in hemodialysis patients. Calcif. Tissue Int. 82(4):278–287, 2008.
Srinivasan, S., D. A. Weimer, S. C. Agans, S. D. Bain, and T. S. Gross. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J. Bone Miner. Res. 17(9):1613–1620, 2002.
Stuermer, E. K., M. Komrakova, S. Sehmisch, M. Tezval, C. Dullin, N. Schaefer, J. Hallecker, and K. M. Stuermer. Whole body vibration during fracture healing intensifies the effects of estradiol and raloxifene in estrogen-deficient rats. Bone 64:187–194, 2014.
Stuermer, E. K., M. Komrakova, C. Werner, M. Wicke, L. Kolios, S. Sehmisch, M. Tezval, C. Utesch, O. Mangal, S. Zimmer, C. Dullin, and K. M. Stuermer. Musculoskeletal response to whole-body vibration during fracture healing in intact and ovariectomized rats. Calcif. Tissue. Int. 87(2):168–180, 2010.
Sun, L. W., Y. B. Fan, D. Y. Li, F. Zhao, T. Xie, X. Yang, and Z. T. Gu. Evaluation of the mechanical properties of rat bone under simulated microgravity using nanoindentation. Acta Biomater. 5(9):3506–3511, 2009.
Wolf, S., P. Augat, K. Eckert-Hübner, A. Laule, G. D. Krischak, and L. E. Claes. Effects of high-frequency, low-magnitude mechanical stimulus on bone healing. Clin. Orthop. Relat. Res. 385:192–198, 2001.
Xie, L., C. Rubin, and S. Judex. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. Appl. Physiol. 104(4):1056–1062, 2008.
Xie, P., Z. Tang, F. Qing, X. Chen, X. Zhu, Y. Fan, X. Yang, and X. Zhang. Bone mineral density, microarchitectural and mechanical alterations of osteoporotic rat bone under long-term whole-body vibration therapy. J. Mech. Behav. Biomed. Mater. 53:341–349, 2015.
Zhang, R., H. Gong, D. Zhu, J. Gao, J. Fang, and Y. Fan. Seven day insertion rest in whole body vibration improves multi-level bone quality in tail suspension rats. PLoS One 9:e92312, 2014.
Acknowledgment
This work is supported by the National Natural Science foundation of China (Nos. 11322223, 11432016, 81471753, and 11272134), the 973 Program (No. 2012CB821202).
Conflict of Interest
The authors have declared that no competing interest exists.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Sean S. Kohles oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Gao, J., Gong, H., Huang, X. et al. Multi-Level Assessment of Fracture Calluses in Rats Subjected to Low-Magnitude High-Frequency Vibration with Different Rest Periods. Ann Biomed Eng 44, 2489–2504 (2016). https://doi.org/10.1007/s10439-015-1532-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1532-z