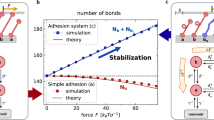
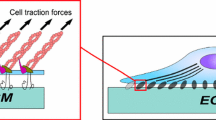
Abstract
A diffusive–stochastic–viscoelastic model is proposed for the specific adhesion of viscoelastic solids via stochastically formed molecular bonds. In this model, it is assumed that molecular-level behaviors, including the diffusion of mobile adhesion molecules and stochastic reaction between adhesion molecules and binding sites, are Markovian stochastic processes, while the mesoscopic deformation of the viscoelastic media is governed by continuum mechanics. Systematic Monte Carlo simulations of this model are used to investigate how competition between the time scales of molecular diffusion, reaction, and deformation creep of the solids may influence the lifetime and dynamic strength of their adhesion. The results reveal that there exists an optimal characteristic time for molecular diffusion corresponding to the longest lifetime and greatest adhesion strength, which is in good agreement with experimentally observed characteristic time scales of molecular diffusion in cell membranes. In addition, the results show that the viscosity of the media can significantly increase the lifetime and dynamic strength, since deformation creep and stress relaxation can effectively reduce the concentration of interfacial stress and increase the rebinding probability of molecular bonds.




Similar content being viewed by others
References
Lo, C.M., Wang, H.B., Dembo, M., et al.: Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152 (2000). https://doi.org/10.1016/S0006-3495(00)76279-5
Discher, D.E., Janmey, P., Wang, Y.: Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005). https://doi.org/10.1126/science.1116995
Peyton, S.R., Putnam, A.J.: Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J. Cell. Physiol. 204, 198–209 (2005). https://doi.org/10.1002/jcp.20274
Gao, H.: Probing mechanical principles of cell–nanomaterial interactions. J. Mech. Phys. Solids 62, 312–339 (2014). https://doi.org/10.1016/j.jmps.2013.08.018
Chaudhuri, O., Gu, L., Klumpers, D., et al.: Hydrogels with tunable stress relaxation. Nat. Mater. 15, 326–334 (2016). https://doi.org/10.1038/nmat4489
Perinpanayagam, H., Zaharias, R., Stanford, C., et al.: Early cell adhesion events differ between osteoporotic and non-osteoporotic osteoblasts. J. Orthop. Res. 19, 993–1000 (2001). https://doi.org/10.1016/S0736-0266(01)00045-6
Giancotti, F.G., Ruoslahti, E.: Integrin signaling. Science 285, 1028–1033 (1999). https://doi.org/10.1126/science.285.5430.1028
Zhao, W., Hanson, L., Lou, H.Y., et al.: Nanoscale manipulation of membrane curvature for probing endocytosis in live cells. Nat. Nanotechnol. 12, 750–756 (2017). https://doi.org/10.1038/nnano.2017.98
Long, M., Zhao, H., Huang, K.S., et al.: Kinetic measurements of cell surface E-selectin/carbohydrate ligand interactions. Ann. Biomed. Eng. 29, 935–946 (2001). https://doi.org/10.1114/1.1415529
Long, M., Goldsmith, H.L., Tees, D.F.J., et al.: Probabilistic modeling of shear-induced formation and breakage of doublets cross-linked by receptor–ligand bonds. Biophys. J. 76, 1112–1128 (1999). https://doi.org/10.1016/S0006-3495(99)77276-0
Mücksch, J., Blumhardt, P., Strauss, M.T., et al.: Quantifying reversible surface binding via surface-integrated fluorescence correlation spectroscopy. Nano Lett. 18, 3185–3192 (2018). https://doi.org/10.1021/acs.nanolett.8b00875
Kramers, H.A.: Brownian motion in a field of force and the diffusion model of chemical reactions. Physica 7, 284–304 (1940). https://doi.org/10.1016/S0031-8914(40)90098-2
Bell, G.I.: Models for the specific adhesion of cells to cells. Science 200, 618–627 (1978). https://doi.org/10.1126/science.347575
Bell, G.I., Dembo, M., Bongrand, P.: Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys. J. 45, 1051–1064 (1984). https://doi.org/10.1016/s0006-3495(84)84252-6
Raible, M., Evstigneev, M., Reimann, P., et al.: Theoretical analysis of dynamic force spectroscopy experiments on ligand–receptor complexes. J. Biotechnol. 112, 13–23 (2004). https://doi.org/10.1016/j.jbiotec.2004.04.017
Bartels, F.W., Baumgarth, B., Anselmetti, D., et al.: Specific binding of the regulatory protein ExpG to promoter regions of the galactoglucan biosynthesis gene cluster of Sinorhizobium meliloti-a combined molecular biology and force spectroscopy investigation. J. Struct. Biol. 143, 145–152 (2003). https://doi.org/10.1016/S1047-8477(03)00127-8
Li, D., Ji, B.: Predicted rupture force of a single molecular bond becomes rate independent at ultralow loading rates. Phys. Rev. Lett. 112, 078302 (2014). https://doi.org/10.1103/PhysRevLett.112.078302
Chen, X., Li, D., Ji, B., et al.: Reconciling bond strength of a slip bond at low loading rates with rebinding. Europhys. Lett. 109, 68002 (2015). https://doi.org/10.1209/0295-5075/109/68002
Evans, E.: Probing the relation between force-lifetime- and chemistry in single molecular bonds. Annu. Rev. Biophys. Biomol. Struct. 30, 105–128 (2001). https://doi.org/10.1002/chin.200151281
Li, F., Redick, S.D., Erickson, et al.: Force measurements of the α5 β1 integrin–fibronectin interaction. Biophys. J. 84, 1252–1262 (2003). https://doi.org/10.1016/s0006-3495(03)74940-6
Merkel, R., Nassoy, P., Leung, A., et al.: Energy landscapes of receptor–ligand bonds explored with dynamic force spectroscopy. Nature 397, 50–53 (1999). https://doi.org/10.1038/16219
Erdmann, T., Schwarz, U.S.: Stability of adhesion clusters under constant force. Phys. Rev. Lett. 92, 108102 (2004). https://doi.org/10.1103/PhysRevLett.92.108102
Erdmann, T., Schwarz, U.S.: Stochastic dynamics of adhesion clusters under shared constant force and with rebinding. J. Chem. Phys. 121, 8997–9017 (2004). https://doi.org/10.1063/1.1805496
Chen, A., Moy, V.T.: Cross-linking of cell surface receptors enhances cooperativity of molecular adhesion. Biophys. J. 78, 2814–2820 (2000). https://doi.org/10.1016/S0006-3495(00)76824-X
Sulchek, T., Friddle, R.W., Noy, A.: Strength of multiple parallel biological bonds. Biophys. J. 90, 4686–4691 (2006). https://doi.org/10.1529/biophysj.105.080291
Sun, L., Cheng, Q.H., Gao, H.J., et al.: Effect of loading conditions on the dissociation behaviour of catch bond clusters. J. R. Soc. Interface 9, 928–937 (2011). https://doi.org/10.1098/rsif.2011.0553
Wang, J., Yao, J., Gao, H.: Specific adhesion of a soft elastic body on a wavy surface. Theor. Appl. Mech. Lett. 2, 014002 (2012). https://doi.org/10.1063/2.1201402
He, S., Su, Y., Ji, B., et al.: Some basic questions on mechanosensing in cell–substrate interaction. J. Mech. Phys. Solids 70, 116–135 (2014). https://doi.org/10.1016/j.jmps.2014.05.016
Li, L., Tang, H., Wang, J., et al.: Rolling adhesion of cell in shear flow: a theoretical model. J. Mech. Phys. Solids 119, 369–381 (2018). https://doi.org/10.1016/j.jmps.2018.07.013
Ward, M.D., Dembo, M., Hammer, D.A.: Kinetics of cell detachment: peeling of discrete receptor clusters. Biophys. J. 67, 2522–2534 (1994). https://doi.org/10.1016/S0006-3495(94)80742-8
Li, L., Yao, H., Wang, J.: Dynamic strength of molecular bond clusters under displacement- and force-controlled loading conditions. J. Appl. Mech. 83, 021004 (2016). https://doi.org/10.1115/1.4031802
Liu, J., Wang, Y.L., Goh, W.I., et al.: Talin determines the nanoscale architecture of focal adhesions. Proc. Natl. Acad. Sci. 17, E4673–E4864 (2015). https://doi.org/10.1073/pnas.1512025112
Li, N., Lu, S.Q., Zhang, Y., et al.: Mechanokinetics of receptor–ligand interaction in cell adhesion. Acta. Mech. Sin. 31, 248–258 (2015). https://doi.org/10.1007/s10409-015-0407-8
Cone, R.A.: Rotational diffusion of rhodopsin in the visual receptor membrane. Nature 236, 39–43 (1972). https://doi.org/10.1002/jss.400010411
Paszek, M.J., Boettiger, D., Weaver, V.M., et al.: Integrin clustering is driven by mechanical resistance from the glycocalyx and the substrate. PLoS Comput. Biol. 5, e1000604 (2009). https://doi.org/10.1371/journal.pcbi.1000604
Smith, A.S., Sengupta, K., Goennenwein, S., et al.: Force-induced growth of adhesion domains is controlled by receptor mobility. Proc. Natl. Acad. Sci. 105, 6906–6911 (2008). https://doi.org/10.1073/pnas.0801706105
Xu, G.K., Qian, J., Hu, J.: The glycocalyx promotes cooperative binding and clustering of adhesion receptors. Soft Matter 12, 4572–4583 (2016). https://doi.org/10.1039/c5sm03139g
Engler, A.J., Sen, S., Sweeney, H.L., et al.: Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). https://doi.org/10.1016/j.cell.2006.06.044
Wang, J., Gao, H.: Clustering instability in adhesive contact between elastic solids via diffusive molecular bonds. J. Mech. Phys. Solids 56, 251–266 (2008). https://doi.org/10.1016/j.jmps.2007.05.011
Qian, J., Wang, J., Lin, Y., et al.: Lifetime and strength of periodic bond clusters between elastic media under inclined loading. Biophys. J. 97, 2438–2445 (2009). https://doi.org/10.1016/j.bpj.2009.08.027
Gao, H., Qian, J., Chen, B.: Probing mechanical principles of focal contacts in cell–matrix adhesion with a coupled stochastic–elastic modelling framework. J. R. Soc. Interface 8, 1217–1232 (2011). https://doi.org/10.1098/rsif.2011.0157
Chen, B., Ji, B., Gao, H.: Modeling active mechanosensing in cell–matrix interactions. Annu. Rev. Biophys. 44, 1–32 (2015). https://doi.org/10.1146/annurev-biophys-051013-023102
Qian, J., Wang, J., Gao, H.: Lifetime and strength of adhesive molecular bond clusters between elastic media. Langmuir 24, 1262–1270 (2008). https://doi.org/10.1021/la702401b
Wang, J., Gao, H.: Size and shape dependent steady-state pull-off force in molecular adhesion between soft elastic materials. Int. J. Fract. 166, 13–19 (2010). https://doi.org/10.1007/s10704-010-9463-z
Zhang, W., Lin, Y., Qian, J., et al.: Tuning molecular adhesion via material anisotropy. Adv. Funct. Mater. 23, 4729–4738 (2013). https://doi.org/10.1002/adfm.201300069
Qian, J., Gao, H.: Soft matrices suppress cooperative behaviors among receptor–ligand bonds in cell adhesion. PLoS ONE 5, e12342 (2010). https://doi.org/10.1371/journal.pone.0012342
Zhang, W., Qian, J., Yao, H., et al.: Effects of functionally graded materials on dynamics of molecular bond clusters. Sci. China-Phys. Mech. Astron. 55, 980–988 (2012). https://doi.org/10.1007/s11433-012-4726-5
Chen, B., Gao, H.: Mechanical principle of enhancing cell–substrate adhesion via pre-tension in the cytoskeleton. Biophys. J. 98, 2154–2162 (2010). https://doi.org/10.1016/j.bpj.2010.02.007
Chen, B., Gao, H.: Motor force homeostasis in skeletal muscle contraction. Biophys. J. 101, 396–403 (2011). https://doi.org/10.1016/j.bpj.2011.05.061
Qian, J., Liu, H., Lin, Y., et al.: A mechanochemical model of cell reorientation on substrates under cyclic stretch. PLoS ONE 8, e65864 (2013). https://doi.org/10.1371/journal.pone.0065864
Xu, G.K., Li, B., Feng, X.Q., et al.: A tensegrity model of cell reorientation on cyclically stretched substrates. Biophys. J. 111, 1478–1486 (2016). https://doi.org/10.1016/j.bpj.2016.08.036
Qian, J., Lin, J., Xu, G.K., et al.: Thermally assisted peeling of an elastic strip in adhesion with a substrate via molecular bonds. J. Mech. Phys. Solids 101, 197–208 (2017). https://doi.org/10.1016/j.jmps.2017.01.007
Wei, Y.A.: Stochastic description on the traction–separation law of an interface with non-covalent bonding. J. Mech. Phys. Solids 70, 227–241 (2014). https://doi.org/10.1016/j.jmps.2014.05.014
Chaudhuri, O., Gu, L., Darnell, M., et al.: Substrate stress relaxation regulates cell spreading. Nat. Commun. 6, 6365 (2015). https://doi.org/10.1038/ncomms7365
Gong, Z., Szczesny, S.E., Caliari, S.R., et al.: Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proc. Natl. Acad. Sci. 115, E2686–E2695 (2018). https://doi.org/10.1073/pnas.1716620115
Li, L., Zhang, W., Wang, J.: A viscoelastic–stochastic model of the effects of cytoskeleton remodelling on cell adhesion. R. Soc. Open Sci. 3, 160539 (2016). https://doi.org/10.1098/rsos.160539
Bihr, T., Seifert, U., Smith, A.S.: Multiscale approaches to protein-mediated interactions between membranes-relating microscopic and macroscopic dynamics in radially growing adhesions. New J. Phys. 17, 083016 (2015). https://doi.org/10.1088/1367-2630/17/8/083016
Erdmann, T., Schwarz, U.S.: Bistability of cell–matrix adhesions resulting from nonlinear receptor–ligand dynamics. Biophys. J. 91, L60–L62 (2006). https://doi.org/10.1529/biophysj.106.090209
Erdmann, T., Schwarz, U.S.: Impact of receptor–ligand distance on adhesion cluster stability. Eur. Phys. J. E 22, 123–137 (2007). https://doi.org/10.1140/epje/e2007-00019-8
Van Kampen, N.G.: Stochastic Processes in Physics and Chemistry. North-Holland Personal Library, Amsterdam (1981)
Wang, J., Huang, Q.: A stochastic description on adhesion of molecular bond clusters between rigid media with curved interfaces. Int. J. Appl. Mech. 7, 1550071 (2015). https://doi.org/10.1142/S1758825115500714
Gillespie, D.T.: A general method for numerically simulating the stochastic time evolution of coupled chemical reactions. J. Comput. Phys. 22, 403–434 (1976). https://doi.org/10.1016/0021-9991(76)90041-3
Gillespie, D.T.: Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 81, 2340–2361 (1977). https://doi.org/10.1063/1.2710253
Zuckerman, D.M., Bruinsma, R.F.: Vesicle–vesicle adhesion by mobile lock-and-key molecules: Debye–Hückel theory and Monte Carlo simulation. Phys. Rev. E 57, 964 (1998). https://doi.org/10.1103/PhysRevE.57.964
Arnold, M., Cavalcanti-Adam, E.A., Glass, R., et al.: Activation of integrin function by nanopatterned adhesive interfaces. ChemPhysChem 5, 383–388 (2004). https://doi.org/10.1002/cphc.200301014
Kusumi, A., Sako, Y., Yamamoto, M.: Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys. J. 65, 2021–2040 (1993). https://doi.org/10.1016/s0006-3495(93)81253-0
Schwarz, U.S., Safran, S.A.: Physics of adherent cells. Rev. Mod. Phys. 85, 1327 (2013). https://doi.org/10.1103/RevModPhys.85.1327
Wu, T., Feng, J.J.: A biomechanical model for fluidization of cells under dynamic strain. Biophys. J. 108, 43–52 (2015). https://doi.org/10.1016/j.bpj.2014.11.015
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 11472119 and 11602099), the Fundamental Research Funds for the Central Universities (Grant lzujbky-2017-ot11), and the 111 Project (Grant B14044).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, K., Li, L. & Wang, J. Diffusive–stochastic–viscoelastic model for specific adhesion of viscoelastic solids via molecular bonds. Acta Mech. Sin. 35, 343–354 (2019). https://doi.org/10.1007/s10409-019-00848-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10409-019-00848-z