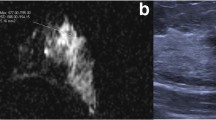
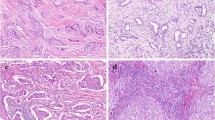
Abstract
Purpose
Tumor-infiltrating lymphocytes (TILs) are known to predict the therapeutic effect in breast cancer. Although a preoperative tissue biopsy can be used to evaluate TILs, TILs that are heterogeneously distributed might require examination of all preoperative tissue biopsy samples. We have recently reported that the TIL ultrasonography (US) score, as determined by characteristic US findings, provides excellent predictive performance for lymphocyte predominant breast cancer (LPBC). We herein aimed to determine whether the preoperative TIL-US score can more accurately predict LPBC than preoperative tissue biopsy.
Methods
We assessed 161 patients with invasive breast cancer that were treated with curative surgery between January 2014 and December 2017. Stromal lymphocytes were examined on preoperative tissue biopsy tissues and surgical pathological specimens. Breast cancer samples with ≥ 50% stromal TILs were defined as pre-LPBC (preoperative tissue biopsy) and LPBC (surgical pathological specimens). Useful factors for predicting LPBC were searched among clinicopathological factors.
Results
The TIL-US score cutoff value for predicting LPBC was 4 points based on the receiver operating characteristic curves (area under the curve: 0.88). Several significant predictors for LPBC were revealed by the undertaken multivariate logistic regression analysis (odds ratios: TIL-US score, 26.8; pre-LPBC, 18.6; HER2, 9.2; all, p < 0.05). The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value were 0.74, 0.89, 0.85, 0.67, and 0.92 for the TIL-US score, respectively, and 0.51, 0.98, 0.87, 0.91, and 0.86 for the pre-LPBC, respectively.
Conclusion
TIL-US scores can predict LPBC preoperatively and are characterized by a significantly high sensitivity and negative predictive value.


Similar content being viewed by others
References
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70.
Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–66.
Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25:1536–43.
Dieci MV, Criscitiello C, Goubar A, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25:611–8.
Perez EA, Ballman KV, Tenner KS, et al. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2016;2:56–64.
Salgado R, Denkert C, Campbell C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO Trial. JAMA Oncol. 2015;1:448–54.
Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–50.
Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–7.
Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13.
Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–91.
Issa-Nummer Y, Darb-Esfahani S, Loibl S, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer–A substudy of the neoadjuvant GeparQuinto trial. PLoS ONE. 2013;8: e79775.
West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13:R126.
Ingold Heppner B, Untch M, Denkert C, et al. Tumor-infiltrating lymphocytes: a predictive and prognostic biomarker in neoadjuvant-treated HER2-positive breast cancer. Clin Cancer Res. 2016;22:5747–54.
Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50.
Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International Tils Working Group 2014. Ann Oncol. 2015;26:259–71.
Denkert C, Wienert S, Poterie A, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29:1155–64.
Swisher SK, Wu Y, Castaneda CA, et al. Interobserver agreement between pathologists assessing tumor-infiltrating lymphocytes (TILs) in breast cancer using methodology proposed by the International Tils Working Group. Ann Surg Oncol. 2016;23:2242–8.
Khoury T, Peng X, Yan L, Wang D, Nagrale V. Tumor-infiltrating lymphocytes in breast cancer: evaluating interobserver variability, heterogeneity, and fidelity of scoring core biopsies. Am J Clin Pathol. 2018;150:441–50.
Crystal P, Strano SD, Shcharynski S, Koretz MJ. Using sonography to screen women with mammographically dense breasts. AJR. 2003;181:177–82.
Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–75.
Weinstein SP, Localio AR, Conant EF, Rosen M, Thomas KM, Schnall MD. Multimodality screening of high-risk women: a prospective cohort study. J Clin Oncol. 2009;27:6124–8.
Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–63.
Tohno E, Umemoto T, Sasaki K, Morishima I, Ueno E. Effect of adding screening ultrasonography to screening mammography on patient recall and cancer detection rates: a retrospective study in Japan. Eur J Radiol. 2013;82:1227–30.
Tohno E, Takahashi H, Tamada T, Fujimoto Y, Yasuda H, Ohuchi N. Educational program and testing using images for the standardization of breast cancer screening by ultrasonography. Breast Cancer. 2012;19:138–46.
Ohuchi N, Suzuki A, Sobue T, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387:341–8.
Chen SC, Cheung YC, Su CH, Chen MF, Hwang TL, Hsueh S. Analysis of sonographic features for the differentiation of benign and malignant breast tumors of different sizes. Ultrasound Obstet Gynecol. 2004;23:188–93.
Costantini M, Belli P, Lombardi R, Franceschini G, Mulè A, Bonomo L. Characterization of solid breast masses: use of the sonographic breast imaging reporting and data system lexicon. J Ultrasound Med. 2006;25:649–59.
Rahbar G, Sie AC, Hansen GC, et al. Benign versus malignant solid breast masses: US differentiation. Radiology. 1999;213:889–94.
Fukui K, Masumoto N, Shiroma N, et al. Novel tumor-infiltrating lymphocytes ultrasonography score based on ultrasonic tissue findings predicts tumor-infiltrating lymphocytes in breast cancer. Breast Cancer. 2019;26:573–80.
Dieci MV, Mathieu MC, Guarneri V, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann Oncol. 2015;26:1698–704.
Çelebi F, Agacayak F, Ozturk A, et al. Usefulness of imaging findings in predicting tumor-infiltrating lymphocytes in patients with breast cancer. Eur Radiol. 2020;30:2049–57.
Jang N, Kwon HJ, Park MH, Kang SH, Bae YK. Prognostic value of tumor-infiltrating lymphocyte density assessed using a standardized method based on molecular subtypes and adjuvant chemotherapy in invasive breast cancer. Ann Surg Oncol. 2018;25:937–46.
Dieci MV, Radosevic-Robin N, Fineberg S, et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International immuno-oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018;52:16–25.
Hamy AS, Pierga JY, Sabaila A, et al. Stromal lymphocyte infiltration after neoadjuvant chemotherapy is associated with aggressive residual disease and lower disease-free survival in HER2-positive breast cancer. Ann Oncol. 2017;28:2233–40.
Acknowledgements
The authors would like to thank Noriyuki Shiroma at the Department of Aki-health Centre for preparing and managing the patient data.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
This study did not include animals. All procedures involving human participants were performed in accordance with the ethical standards set by our Institutional Research Committee (IRB No. 1166) and the 1964 Declaration of Helsinki (and its later amendments) or by comparable ethical standards.
Informed consent
The requirement for formal consent was waived because of the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kanou, A., Masumoto, N., Fukui, K. et al. The tumor-infiltrating lymphocyte ultrasonography score can provide a diagnostic prediction of lymphocyte-predominant breast cancer preoperatively. J Med Ultrasonics 49, 709–717 (2022). https://doi.org/10.1007/s10396-022-01240-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-022-01240-4