Abstract
Purpose
Blood flow reduction after initiation of lenvatinib therapy may not always indicate tumor necrosis. This study aimed to compare the blood flow detectability of contrast-enhanced ultrasonography (CEUS), contrast-enhanced computed tomography (CT), and contrast-enhanced magnetic resonance imaging (MRI) in hepatocellular carcinoma (HCC) during lenvatinib therapy.
Methods
A total of 12 cases underwent CEUS and contrast-enhanced CT/MRI within 2 weeks during lenvatinib therapy. Vascularity on CEUS and CT/MRI was compared.
Results
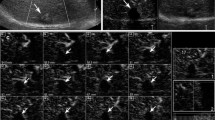
At the time of CEUS examination, the median period from the start of lenvatinib was 227 ± 210 (31–570) days. CEUS showed hyperenhancement in eight cases (66.7%), hypoenhancement in two cases (16.7%), and no enhancement in one case (8.3%), while CT/MRI showed hyperenhancement in one case (8.3%), ring enhancement in three cases (25.0%), and hypoenhancement in eight cases (66.7%) (p = 0.007). Transarterial chemoembolization (n = 3), radiofrequency ablation (n = 2), and stereotactic body radiation therapy (n = 2) were performed after blood flow detection by CEUS.
Conclusions
The viability of the HCC should be confirmed using CEUS when contrast-enhanced CT/MRI reveals lesion hypoenhancement during lenvatinib therapy.






Similar content being viewed by others
References
Kudo M, Izumi N, Sakamoto M, Liver Cancer Study Group of Japan, et al. Survival analysis over 28 years of 173,378 patients with hepatocellular carcinoma in Japan. Liver Cancer. 2016;5:190–7.
Zschäbitz S, Grüllich C, Lenvantinib A. Tyrosine kinase inhibitor of VEGFR-3, FGFR 1–4, PDGFRα, KIT and RET. Recent Results Cancer Res. 2018;211:187–98.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73.
Hiraoka A, Kumada T, Kariyama K, Real-life Practice Experts for HCC (RELPEC) Study Group and the HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan), et al. Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: multicenter analysis. Hepatol Res. 2019;49:111–7.
Kunimoto H, Shakado S, Tanaka T, et al. Reduction in tumor stain at 2 weeks after treatment initiation is a predictor of the efficacy of lenvatinib in patients with unresectable hepatocellular carcinoma. Oncology. 2020;98:779–86.
Koulakian H, Allaham W, Vilgrain V, et al. Non-measurable infiltrative HCC: is post-contrast attenuation on CT a sign of tumor response? Eur Radiol. 2019;29:4389–99.
Kuroda H, Abe T, Fujiwara Y, et al. Change in arterial tumor perfusion is an early biomarker of lenvatinib efficacy in patients with unresectable hepatocellular carcinoma. World J Gastroenterol. 2019;25:2365–72.
Kamachi N, Nakano M, Okamura S, et al. Evaluating the therapeutic effect of lenvatinib against advanced hepatocellular carcinoma by measuring blood flow changes using contrast-enhanced ultrasound. Cancer Rep (Hoboken). 2021;5:e1471.
Numata K, Fukuda H, Miwa H, et al. Contrast-enhanced ultrasonography findings using a perflubutane-based contrast agent in patients with early hepatocellular carcinoma. Eur J Radiol. 2014;83:95–102.
Sugimori K, Numata K, Okada M, et al. Central vascular structures as a characteristic finding of regenerative nodules using hepatobiliary phase gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid-enhanced MRI and arterial dominant phase contrast-enhanced US. J Med Ultrason. 2017;44:89.
Moschouris H, Malagari K, Gkoutzios P, et al. Intermediate and advanced hepatocellular carcinoma treated with the antiangiogenic agent sorafenib. Evaluation with unenhanced and contrast-enhanced ultrasonography. Med Ultrason. 2012;14:87–94.
Kaufmann S, Thaiss WM, Schulze M, et al. Prognostic value of perfusion CT in hepatocellular carcinoma treatment with sorafenib: comparison with mRECIST in longitudinal follow-up. Acta Radiol. 2018;59:765–72.
Kanda Y. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Lassau N, Koscielny S, Chami L, et al. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification–preliminary results. Radiology. 2011;258:291–300.
Zocco MA, Garcovich M, Lupascu A, et al. Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: the role of dynamic contrast enhanced ultrasound. J Hepatol. 2013;59:1014–21.
Sugimoto K, Moriyasu F, Saito K, et al. Hepatocellular carcinoma treated with sorafenib: early detection of treatment response and major adverse events by contrast-enhanced US. Liver Int. 2013;33:605–15.
Shiozawa K, Watanabe M, Ikehara T, et al. Evaluation of sorafenib for advanced hepatocellular carcinoma with low α-fetoprotein in arrival time parametric imaging using contrast-enhanced ultrasonography. J Med Ultrason. 2017;44:101–7.
Lamuraglia M, Escudier B, Chami L, et al. To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: pilot study using dynamic contrast-enhanced Doppler ultrasound. Eur J Cancer. 2006;42:2472–9.
Ang J, Hu L, Huang PT, et al. Contrast-enhanced ultrasonography assessment of gastric cancer response to neoadjuvant chemotherapy. World J Gastroenterol. 2012;18:7026–32.
Kumagawa M, Matsumoto N, Miura K, et al. Correlation between alterations in blood flow of malignant lymphomas after induction chemotherapies and clinical outcomes: a pilot study utilising contrast-enhanced ultrasonography for early interim evaluation of lymphoma treatment. Clin Radiol. 2021;S0009–9260:00089–91.
Cyran CC, Kazmierczak PM, Hirner H, et al. Regorafenib effects on human colon carcinoma xenografts monitored by dynamic contrast-enhanced computed tomography with immunohistochemical validation. PLoS ONE. 2013;8: e76009.
Kim MJ, Choi JI, Lee JS, et al. Computed tomography findings of sorafenib-treated hepatic tumors in patients with advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1201–6.
Liu LP, Dong BW, Yu XL, et al. Focal hypoechoic tumors of Fatty liver: characterization of conventional and contrast-enhanced ultrasonography. J Ultrasound Med. 2009;28:1133–42.
Muñoz NM, Minhaj AA, Maldonado KL, et al. Comparison of dynamic contrast-enhanced magnetic resonance imaging and contrast-enhanced ultrasound for evaluation of the effects of sorafenib in a rat model of hepatocellular carcinoma. Magn Reson Imaging. 2019;57:156–64.
Zhu AX, Holalkere NS, Muzikansky A, et al. Early antiangiogenic activity of bevacizumab evaluated by computed tomography perfusion scan in patients with advanced hepatocellular carcinoma. Oncologist. 2008;13:120–5.
Coolens C, Driscoll B, Moseley J, et al. Feasibility of 4D perfusion CT imaging for the assessment of liver treatment response following SBRT and sorafenib. Adv Radiat Oncol. 2016;1:194–203.
Acknowledgements
The authors thank Editage (https://www.editage.jp/) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Owing to the retrospective nature of this study, informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Matsumoto, N., Ogawa, M., Kaneko, M. et al. Contrast-enhanced ultrasonography for blood flow detection in hepatocellular carcinoma during lenvatinib therapy. J Med Ultrasonics 49, 425–432 (2022). https://doi.org/10.1007/s10396-022-01204-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-022-01204-8