Abstract
Aim
The vCare system is a virtual coach that involves physical and cognitive telerehabilitation and a daily life monitoring system. This pilot study aims to evaluate the vCare pilot test for Parkinson’s disease (PD) patients and to analyze the usability and the satisfaction level of patients and their quality of life (QoL).
Subject and methods
Twenty PD patients were randomized, 10 patients into the vCare group focused on personalized home telerehabilitation [motor and cognitive rehabilitation (4 days/week for 4 months)], while the control group (10 patients) continued the clinical standard at the clinic. A pre-post clinical evaluation and a cost-utility study were performed.
Results
Repeated measures ANOVA showed significant improvement in the PD vCare group compared to the control group (p<.05). Specifically, the PD vCare group showed significant improvement in cognition (p=.016), and QoL dimensions of mobility (p=.008), self-care (p=.008), daily activities (p=.010) and pain/discomfort (p=.004) at post-treatment. vCare PD patients showed high adherence to the vCare system (90.5-100%). Costs per patient in the control group were higher (€5,108.26) than in the vCare group (€2,243.07).
Conclusion
The PD vCare group significantly increase their QoL, cognition, motor symptoms, and daily life activities compared to the control group. Patients showed high adherence to the vCare coach, the care plan, rehabilitation, and devices. The vCare system seems to be an optimal and cost-effective tool for telerehabilitation in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Rehabilitation is built by measures that have the main objective to improve function and prevent complications in a person with a disability or disease (Bickenbach 2011). Rehabilitation can make a real difference in a patient’s life quality (QoL). However, according to a systematic review performed recently (Del Pino et al. 2022), multidisciplinary rehabilitation is not included for neurodegenerative diseases in the public health system in most countries. Even when rehabilitation is essential in the recommended treatment of some diseases it is often interrupted during the process when the patients do not have the guidance needed to perform it at home (Kyriazakos et al. 2020). Therefore, telerehabilitation is a way to provide remote rehabilitation to patients. Telerehabilitation has the objective to improve the QoL of patients using technology and communication tools that make it possible to perform personalized treatment at the patient's home (Rosen 1999). Cognitive rehabilitation improves neuropsychological functioning, improving cognitive domains such as memory and processing speed in patients with Parkinson's disease who are cognitively impaired (Peña et al. 2014).
Technology can provide more accurate data about the patient`s general health condition improving the personalization of the treatment including the rehabilitation by considering the differences between patients with the same disease (Warraich et al. 2018). Health professionals and patients consider technology useful for the rehabilitation process highlighting the importance of pursuing the improvement of the patient-physician relationship and communication (Seregni et al. 2021). A virtual coach could be of great help for health promotion in home environments. The virtual coach can help users to lead a healthier lifestyle and follow an activity program according to their needs, in order to increase functional recovery and reduce the risk of new events (Kyriazakos et al. 2020). Specifically, this applies to neurological diseases such as Stroke or Parkinson’s disease (PD). It has been proved that telerehabilitation is a cost-effective tool in neurological and cardiological diseases (Del Pino et al. 2022).
PD is a chronic, slow, and progressive disease that presents motor and non-motor symptoms, most of the healthcare systems do not treat it through physical or cognitive rehabilitation (World Health Organization 2017). The standard care usually is based on the follow-up of neurological clinical care once or twice per year. Therefore, the use of technology to measure and follow up on PD symptoms could be useful for clinicians, allowing them to have objective information about the symptoms and the activities of daily living for longer periods of time even when the patient is at home (World Health Organization 2017). The data collected by technological devices has the advantage of providing information that complements the one obtained during the clinical evaluation and has more ecological validity (Morgan et al. 2020).
The vCare system (https://vcare-project.eu/) is an international multicenter project with a technological and clinical base with the participation of 12 centers from 7 European countries, including 4 clinical centers, whose objective is to create a virtual training platform based on an intelligent information and communication technology environment for rehabilitation in neurological and cardiological diseases associated with aging, our aim is to focus on PD. vCare offers personalized home rehabilitation with a virtual coach system using an intelligent avatar that assists and encourages patients to perform activities that promote QoL considering physical, cognitive, mental, and social aspects of the patient’s life. The vCare avatar interacts with patients to guide them through the different activities required for their rehabilitation program, the use of other technologies such as presence sensors and health monitoring devices is useful to collect the information needed to follow up on the patient’s progress and personalize the rehabilitation program (Seregni et al. 2021). Therefore, the primary aim of this study was to perform a pilot test using the vCare system for PD patients and to evaluate the improvement of the QoL, the reductions of risk factors, the adherence to home care and rehabilitation plan, and the personalization and health promotion, as well as to assess the usability and satisfaction level of using vCare. The secondary aim was to carry out a cost-utility analysis of the effectiveness of the vCare system in PD patients.
Material and methods
Study design and participants
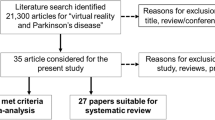
This pilot study is conceived as a prospective interventional experimental study, the main purpose of which is supportive care. A total of 20 participants with PD were randomly assigned to the intervention (vCare group) or control group. A total of 10 patients were assigned to the intervention group and performed telerehabilitation with vCare system during a 4-month period. In addition, 10 patients were assigned to the control group with the same demographic characteristics who received the standard care at the clinic (Fig. 1). Participants were recruited through the Department of Neurology at Cruces University Hospital (Barakaldo, Spain). The study protocol was approved by the Basque Research Ethics Committee (CEIm-E, code: PS2021041). All participants gave written informed consent prior to their participation in the study, in accordance with the tenets of the Declaration of Helsinki.
Patients with PD fulfilled Parkinson’s UK Brain Bank criteria for the diagnosis of PD (Hughes et al. 1992). Other inclusion criteria: (1) Age > 60 years; (2) Patients with scores higher than 60% on the daily life activities scale of Schwab and England; (3) Presence of motor fluctuations perceived by the patient; (4) Hoehn and Yahr stage between 1 and 3; (5) Willingness to interact with technological devices; (6) Internet connection at home; (7) TV screen with HDMI port at home. The exclusion criteria were as follows (1) Patients diagnosed with atypical Parkinsonism, dementia, or other chronic diseases such as heart failure, severe lung, or liver problems; (2) bedridden patients; (3) Patients with severe psychiatric problems such as hallucinations or major depression; (4) Patients with poor adherence prior to pharmacological or rehabilitative treatment; (5) Unable to understand and comply with protocol and/or give informed consent.
Clinical and usability evaluation
The clinical evaluation protocol consisted of measuring: (1) QoL through the Euro Quality of Life 5 Levels (EQ5D-5L: mobility, self-care, daily activities, pain/discomfort, anxiety/depression, and perceived health) (Ramos-Goñi et al. 2018); (2) Cognitive general status with the Montreal Cognitive Assessment (MoCA) (Nasreddine et al. 2005); (3) Motor symptoms with the Unified Parkinson’s Disease Rating Scale (UPDRS) part I, II, III, and IV (Fahn et al. 1987); (4) Functional disability associated with PD and the progression of the disease with the Modified Hoehn and Yahr scale (H&Y) (Hoehn and Yahr 1976); (5) Capabilities for performing activities of daily living with the Schwab and England Activities of Daily Living (ADL) (Gillingham and Donaldson 1967).
The usability and satisfaction evaluations were only performed for the vCare group: (1) System usability scale (SUS) (Brooke 1986); (2) User Experience Questionnaire (UEQ) (Rauschenberger et al. 2013) evaluates 6 domains that include attractiveness, perspicuity, efficiency, dependability, stimulation, and novelty of the vCare system. The range of the scales is between -3 (“horribly bad”) and *3 (“extremely good”). Values between -0,8 and 0.8 represent a neutral opinion of the vCare system; values >0,8 represents a positive evaluation; while <-0,8 means a negative evaluation of the vCare system.
Procedure
The vCare project consisted of 5 phases: 1. Definition of architecture/interface and technical preparation phase. The first stage was to define the architecture and interface of the system; 2. Preliminary phase. This phase consisted of modeling narratives using knowledge representation methods, such as pathways, profiles (diseases, users, coach, treatment, activity, performance, and environment), and ontologies depending on the disability/ impairment to be recovered. The activity was performed according to a process-driven approach, addressing different care pathways and related supporting services; 3. Tech Labs phase. This phase consisted of performing the test setup including test preconditions, test conducted execution, test validation dimensions, impressions of medical partners during and after the tests, and finally a comprehensive test summary. In addition to the functional requirements validated by the test cases, the non-functional requirements were also addressed; 4. Living Labs phase. The Living Lab Phase included PD patients recruited at Biocruces Bizkaia Health Research Institute/Cruces University Hospital (ethical committee approval Code: PS2020039) and had the aim to assess the vCare system usability of the devices and the satisfaction level of the 20 PD patients involved in the present study for a two-week period in a ‘controlled environment’. This controlled environment was an apartment intended exclusively for the living lab, which was provided with all the necessary equipment to perform the vCare telerehabilitation and monitoring system, where patients behaved like at their own homes (Seregni et al. 2021); 5. Pilot phase. Finally, the pilot test was conducted at the patients homes for 4 months. Patients were assigned to the intervention or control group by randomization (not masked) in 5 blocks of 4 subjects for each block. Figure 2 shows the flow diagram. All patients performed a clinical evaluation (pre- and post-intervention), and the clinicians designed the personalized telerehabilitation program for each patient included in the PD vCare group. However, the control group for this study did not perform traditional rehabilitation, just the clinical health standard at the clinic.
Rehabilitation
Definition of the rehabilitation treatment
The vCare telerehabilitation followed a routine of clinical condition monitoring, risk prevention, and motor and cognitive rehabilitation synchronized by an intelligent system with an avatar, which scheduled the rehabilitation based on the plan established by the clinicians responsible for the patient. The interaction between the patient and the avatar was done through a television and a tablet installed in the patient's home. The vCare system's intelligent algorithms adapted the rehabilitation routine set by the responsible clinicians to the patient's situation and continuously reported on the patient's clinical condition and response to rehabilitation.
Before defining the home telerehabilitation pathway provided through the vCare solution (Gand et al. 2021; Gißke et al. 2022) (for the vCare group), clinicians gathered the user’s personal data into the Professional Portal (KIOLA), and the client ID, the correspondent user, and password associated with the Gmail account was created for each user. These credentials were required to associate each user in the Keycloak and in the Knowledge representation framework (KRF) server. Finally, the clinical team defined the motor and cognitive status of the patient, by entering the most relevant clinical information collected during the pre-intervention evaluation.
The cognitive and motor telerehabilitation activities consisted on the serious games suite REHABILITY package (www.rehability.me) and included: (1) Attention; (2) Executive Functions; (3) Attention+ Executive Functions; (4) Mobility; (5) Strengthening; (6) Coordination; (7) Dexterity; (8) Speed; (9) Motor Control; (10) Postural Control; (11) Balance; (12) Endurance; (13) Rhythm. The clinical team the motor and cognitive telerehabilitation treatment to be performed at home.
The telerehabilitation for PD patients performed by the vCare system followed the same structure as the traditional rehabilitation at the clinic. The PD vCare group performed the motor and cognitive rehabilitation sessions 4 times a week (2 days for motor sessions and 2 days for cognitive sessions). The games selected, the time per game and the difficulty for each game were different and personalized for each patient. The motor session was performed through a TV connected with a 3-dimensional (3D) camera and with a mini-PC unit while the cognitive session was done with a tablet; both platforms were connected to the internet, to vCare dedicated cloud servers, and the vCare app (the avatar) installed in the tablet. Each session lasted approximately 30 minutes, 20 minutes at the minimum and 45 minutes at the maximum. Traditional rehabilitation is guided by a clinician (physiotherapist and neuropsychologist) while in the vCare system, the virtual coach guided the patient once the clinician has designed in the platform the rehabilitation plan for each patient.
Before performing the rehabilitation, the patient had to open the vCare app to interact with the virtual coach. Before the telerehabilitation session, patients were evaluated by the vCare avatar with a fatigue questionnaire (Herlofson and Larsen 2002), and depending on the score, a specific telerehabilitation session was personally designed for each patient. For example, if the patient did not present fatigue, the patient was invited by the virtual coach to participate in the scheduled sessions via notifications on the tablet, and during the session, it provided the user with postural feedback in real-time to correct any posture compensation. However, if the patient presented with a fatigue score, depending on the degree of fatigue, the vCare avatar coach suggested resting and/or a shorter telerehabilitation session.
The motor serious games proposed by Virtual Coaching were task-oriented, through gestures performed while sitting or standing, to stimulate the body districts and (neuro) motor functions in agreement with the motor deficit shown by the patient during clinical evaluation. The cognitive serious games included the following cognitive domains: selective, divided, and sustained attention, visual-spatial planning, abstraction, categorization, working memory and calculation. In addition to the home-based motor activities and home-based cognitive games, each PD patient vCare system was assigned electronic learning (E-learning), coaching for an active lifestyle, and falls prevention programs. Subjects had available a library of educational and informative multimedia content about rehabilitation, including the management of home risks, information about the disease, the motor and non-motor symptoms, and information related to different treatments for PD. This material was used within the vCare app installed on the tablet supplied to all patients.
PD vCare group monitoring
The monitoring task was performed through different sensors: movement and presence sensors from MYSPHERA (www.mysphera.com), the smart band (Beurer AS97 or XIAOMI band) recorded the daily number of steps and the heart rate, and the STAT-ON device (PD Holter, www.statonholter.com) recorded the PD motor symptoms such as freezing of gait, number of steps, movement, bradykinesia, time in OFF and ON state, and dyskinesias. The movement and presence sensors and the smart band were installed at the patient’s home for the whole telerehabilitation period. However, at the house of the patients PD01 and PD02, there seemed to be a technological issue with the connection of the devices and just the sensor of the bedroom was recording the information and only for 2 months. The STAT-ON device was used during the first and last week of the Pilot study (7 days each week). In addition, the clinical staff facilitated the patients with a telephone number to contact if needed. Almost every week, contact was maintained with the vCare PD patients to verify that everything was working correctly and/or if there were any technical issues to solve them.
Cost-utility analysis
Data related to QoL were collected from the EQ5D-5L before and after the intervention in both groups. Regarding the utilities, values from 0 to 1 have been obtained for the health states provided by the patients through the EQ-5D-5L quality of life survey, following the methodology published by Ramos-Goñi et al. (2018). The patient pathway for traditional rehabilitation and the one that would be followed with vCare were reviewed and validated (Ramos-Goñi et al. 2018). With this information, the use and consumption of resources for both types of rehabilitation were quantified. Information on the unit cost of each of these resources was extracted from the Ezkerraldea-Enkarterri-Cruces Integrated Healthcare Organization (EEC IHO) Cost per Patient Information System (Osakidetza). The costs per patient of both rehabilitation (regular rehabilitation at the clinic versus vCare telerehabilitation) were calculated using the following resources from the hospital information system: face-to-face neurological, neuropsychological, and motor consultation (first and successive), telephone consultation (first and successive), motor and cognitive rehabilitation, neuropsychological evaluation; and from the vCare system costs: avatar voice, Rehability (i-maginary), vCare maintenance, devices, design of motor and cognitive sessions, installation and uninstallation of devices. Both types of rehabilitation include three consultations at the beginning and at the end of the process, which lasts 4 months. Motor and cognitive rehabilitation (both, telerehabilitation or standard care) were performed 4 times a week for 12 weeks. Both rehabilitations included a face-to-face clinical, neurological, neuropsychological, and motor pre-post evaluation (Time 0 and Time 1). During the traditional rehabilitation time, no face-to-face consultation is needed while during the vCare telerehabilitation, a telephone follow-up consultation is performed 2 times a month.
The cost of subsequent consultations is half the cost of the first consultation, according to corporate criteria. The cost of telephone consultations is 0.4 of the cost of a face-to-face consultation, according to corporate criteria. The cost of motor rehabilitation corresponds to neurological rehabilitation sessions of the EEC IHO Rehabilitation Service. The cost of cognitive rehabilitation corresponds to the cost of a neuropsychological consultation.
Statistical analysis
Demographic and clinical data of PD patients (vCare and control group) were collected and recorded on an Excel sheet by the clinical team. Statistical analyses were carried out using IBM SPSS Statistics for Windows, version 20.0 (IBM-SPSS, Armonk, New York). The Kolmogorov-Smirnov test was performed to determine whether the study variables followed a normal distribution. Descriptive analyses were performed for the mean and standard deviation of each variable at time 0 (baseline, pre-intervention) and time 1 (post-intervention). Repeated measures ANOVA was performed to test “groupxtime” interaction between PD vCare group and the control group at post-treatment compared to pre-treatment. Moreover, the Wilcoxon test for intergroup differences was performed. Regarding the data extracted from the STAT-ON device, a t-test for paired samples was used since the STAT-ON recorded a large number of measurements for each patient and compared the differences between the pre-and post-intervention of these groups of measurements. The data extracted by the vCare app was facilitated by the technical team in charge of that task [Forschungszentrum Informatik (FZI), Austrian Institute of Technology (AIT), and IMAGINARY SRL (IMA)]. In addition, correlations between MoCA, UPDRS, EuroQol-5D, data extracted from the STAT-ON device, and data extracted by the vCare app were analysed with Spearman's bivariate correlation coefficient. Statistical significance was set at p<0.05 (two-tailed). The outcome measures were analysed according to the objectives established for the PD pilot.
Regarding the monitorization of motor symptoms through the STAT-ON Holter, each parameter of interest was compared using the mean measurement per day against two groups, pre-versus post-measurements. Each parameter was calculated using 3 strategies: (i) against all days, (ii) against the 3 worst days (the three days with the most hours in OFF), and (iii) against the 3 best days (the three days with the most hours in ON). Supplementary material (Table 1) presents the mean difference (pre minus post) and the p-value of the pre vs. post sets using the t-test. The measure per day is obtained by averaging the measure over the corresponding day. In the case of Hours OFF, OFF+INT, ON, ON+INT, dyskinesia (Dysk) and Gray] the measure per day is the percentage of monitored time in which the patient has been diagnosed with that state. Freezing of gait (FoG) episodes are counted by aggregating them by day. Finally, the thresholds (Upper: Threshold_HI and Lower: Threshold_LO) are common for all monitoring and therefore the table presents only the difference (pre minus post) between the thresholds of the two monitoring.
Lastly, the cost-utility was performed with the Monitoring and Assessment Framework for the European Innovation Partnership on Active and Healthy Ageing (MAFEIP) tool following the European recommendations, including the costs of both rehabilitations and the effectiveness measured in quality-adjusted life years (QALY), given a threshold of €20,000 per QALY (Vallejo-Torres et al., 2018).
Results
PD Patient’s characterization and monitoring duration
Twenty patients were enrolled in the PD vCare pilot, 10 PD patients were randomly included in each group (PD vCare group and control group). 70% of PD patients were males in both groups. The mean age of the PD vCare group was slightly younger (64.5±7.9) than the control group (69.1±3.5) (p=.105), although there were no statistically significant differences in age or education (PD vCare group education: 13.7±4.4; control group: 12±4.5; p=.684) between groups. PD vCare patients were monitored for a 4-month period.
Medical outcomes
Clinical results
Repeated measures ANOVA timexgroup interaction revealed statistically significant differences between groups, showing improvements in the PD vCare group compared to the control group in diverse domains, including QoL levels, mobility (F=-21.8; p < .001), self-care (F=-10.7; p=.005), daily activities (F=-13.5; p=.002) and pain and Discomfort (F=-45.3; p < .001), but also in daily activities (F=-6.3; p=.026) assessed with the daily life activities scale of Schwab and England, and marginally significant differences in MoCA (F=-4.5; p=.051). In addition, patients before performing the clinical evaluation commented verbally about feeling better and that their subjective processing of speed was faster. However, there were no differences in anxiety/depression dimension and neither in their perceived health.
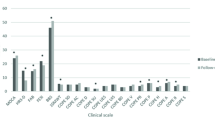
Regarding intra-group differences, the PD vCare group showed statistically significant differences at post-treatment compared to pre-treatment, showing improvements in general cognitive status measured with MoCA (z=-2.4; p=.016) (Table 1), in QoL dimensions of mobility (z=-2.6; p=.008), self-care (z=-2.6; p=.008), daily activities (z=-2.6; p=.010), and pain/discomfort (z=-2.8; p=.004), finding a better QoL in the PD vCare group, at post-intervention compared to the pre-intervention. Table 1 and Fig. 3 show the PD results of QoL of the PD vCare group and the control group. Furthermore, the PD vCare group showed marginally significant differences in daily activities measured with ADL (z=-1.9; p=.058) at post-treatment compared to pre-treatment. On the contrary, the control group showed no significant differences after intervention in any of the domains assessed.
Reports activities in the pilot at home
Monitorization of PD motor symptoms
PD vCare group wore the STAT-ON Holter for 7 days the first week of using vCare and another 7 days after finishing the vCare intervention) measuring the mean of walking minutes, mean of the number of steps per day, gait fluidity, time in OFF, time in ON, dyskinesia, bradykinesia, and the number of freezing of gate. Regarding the monitorization of steps, it was measured with the STAT-ON Holter. The mean of walking minutes per day from the PD vCare group was 69.0±31.4 and did 7437.0±3981.3 steps per day at time 0 (pre-intervention) and walked 63.5±26.7 minutes per day and did 6989.1±3361.1 steps per day at time 1 (post-intervention). No significant differences were found between the number of steps per day recorded and the mean of minutes walking per day. However, significant improvements in patients’ gait fluidity, step length (in meters), fewer hours in OFF, more hours in ON, and less dyskinesia between pre-post intervention, taking into account that no modification of the treatment was done during the intervention period.
Presence monitorization of PD
Patients from the PD vCare group were monitored by presence and movement sensors from MYSPHERA. The location distribution per patient and months monitored is shown as supplementary material (Fig. 4). The data was extracted from an InfluxDB database hosted by each smart-home device (a Raspberry pi 4b). The sensors recorded the information from February to May in February (45.6%) and March (43.8%) patients spent most time in the bedroom. However, during April (41.4%) the place where the monitorization registered more time was the bathroom, and during May (37.7%) the living room, which was also the second most common place where the patients spent their time the other months (26.9%), March (27.2%) and April (32.6%). On the other hand, the kitchen was the place with less presence of the patients registered (February=14.9%, March=9.1%, April=7.1%, and May=11.8%).
Usability and satisfaction level of vCare PD group. A System usability scale (SUS) scores for the PD vCare group at post-intervention. The red horizontal line indicates the limit of acceptability (i.e., 68 points). B User Experience Questionnaire (UEQ) results of PD vCare group pre-post intervention
Parameters registered by the PD vCare system
The vCare system registered the accesses to the vCare app, the e-learning videos watched, the interactions between the vCare avatar and the patient, the activities overview, and the pathway adaptation per patient. Table 2 shows these data (active weeks, mean, or percentage of adherence). In general, all PD patients had high adherence to the vCare system, most of them with 100% of adherence, except for the e-learning videos and the interactions with the vCare avatar. The patients were active with the vCare application for 4 months. Regarding the “e-learning videos”, this metric represents the adherence to watching the videos recorded for PD disease that included an introduction to the disease, an explanation of motor and non-motor PD symptoms, first-line and second-line treatment for PD, possible risk at the kitchen, and an explanation of the project. Three patients watched all the videos while 2 patients did not use this tool. It was not mandatory to watch the videos, but they were recommended, as it helps to have a better knowledge of the disease and to better understand and recognize the symptoms. The “VC patient interactions” were measured by taking into account the total number of reminders by the patient and the number of responses by the patient either by feedback or by answering the questionnaires. It was observed that 7 out of 10 patients showed 100% adherence with the avatar and only one patient showed low adherence, with 22.2%. Regarding the “activity overview” includes the activities planned and tested for each patient (home-based motor activities, home-based cognitive games, coaching for an active lifestyle, fall prevention, and e-learning). The “adherence” metric represents when the vCare avatar interacted with the patient and the patient answered it. This metric showed the lowest adherence, being the highest 61.3% of adherence. Considering all patients, the average adherence was 25.2%. Finally, the “pathway adaptation” showed a complete adherence to all the patients (100%); for each pathway, the avatar was able to adapt the activity, such as decreasing or increasing the time of the game or suggesting an outdoor walk, among others.
Table 2 also shows the total and mean scores for motor and cognitive telerehabilitation sessions, the prescribed sessions, the completed sessions, the played minutes, and the percentage of adherence to the vCare motor and cognitive games. The mean adherence of the PD patients was 96.5% to vCare motor games and 96.6% to vCare cognitive games, meaning that patients had high adherence to both. The only patient that had an adherence of 90% was a patient that had technical problems with the camera of the vCare solution.
Usability and satisfaction
No significant differences were found in the usability and satisfaction results between the pre-post intervention data of the PD vCare group. Figure 5 shows the SUS data obtained by patients enrolled in the PD vCare group. Eight PD patients out of 10 patients evaluated the vCare system with a SUS score greater than 68 points. In particular, six PD patients evaluated the usability as “best imaginable”, with a score higher than 85. Remarkably, one patient rated the usability with an almost maximum SUS score, of 97.5. Just one patient rated the usability of the vCare lower than 60 (55 points). These results confirmed the intuitiveness and the overall ease of use of the vCare system that has already been assessed in the previous experimental phase (i.e., Living Lab Phase). However, in the case of the Pilot Phase, the added value is that overall good usability of the vCare system was found even within the home context, without the constant and certain assistance of the therapist. Regarding the UEQ, the vCare PD patients group highlighted the perspicuity of the vCare system, its stimulation, and the novelty of the system.
Improvement indicators
According to the PD outcome measures, Table 3 shows the PD vCare goals, the indicators, and the outcomes achieved, finding that the PD vCare pilot achieved the key performance indicators proposed. There was an improvement in the primary outcome, the QoL of PD patients, and the secondary outcomes: reduction of risk factors, adherence to the home care and rehabilitation plan, and personalization and health promotion. The vCare PD patients increase their QoL (higher than 50% of the increase), 62.5% of patients significantly improved their fluidity, related to gait and bradykinesia, presented high adherence to the motor and cognitive rehabilitation (>90%), number of accesses to e-learning, to the home care and rehabilitation plan, and vCare was able to make adaptations to the PD pathways.
Relation between PD symptoms, quality of life and data extracted from the STAT-ON device and the vCare app at post-telerehabilitation
Related to QoL, increased self-care was related to increased mobility (EQ5D-5L, Rho=.8; p=.006) and higher average time performing the motor games (Rho= .8; p=.010) (vCare Rehability app). Lower time in ON status (measured by the STAT-ON) was correlated to higher pain (EQ5D-5L scale) (Rho=-.7; p=.040). On the other hand, increased dyskinesia was correlated with more time in ON status (STAT-ON) (Rho=.8; p=.010) and with a higher score in the UPDRS IV (Rho=.8; p=.028). In addition, higher QoL related to anxiety and depression (EQ5D-5L were related to more daily steps (Rho=.8; p=.007) and minutes walked (Rho=.8; p=.023). Related to the OFF status (STAT-ON), more time in OFF was correlated with a lower score in the motor rehabilitation performed in the vCare Rehability app (Rho=-.8; p=.010). Lastly, a positive correlation was found between daily life activities (UPDRS II) and motor symptoms (UPDRS III) (Rho=.8; p=.007). Besides, a higher score in the UPDRS IV was related to the worst perception of their own general health reported by the patient in the EQ5D-5L scale (Rho=-.8; p=.004).
Cost-utility analysis
The cost of 3 months of traditional rehabilitation was €5108.26 while the use of the vCare system for 3 months cost €2243.07. There were no implementation or unique costs (One-off-costs) and all resource consumption was included in the cost per patient. Social or additional costs were not considered. The difference in cost is based on the fact that the physical presence of the professional was not necessary when performing rehabilitation using the vCare system since the professionally designed and configured rehabilitation program using the KIOLA platform and the games, sessions, difficulty and time to perform the exercises were defined in REHABILITY. In traditional rehabilitation, the professional was physical with the patient while the rehabilitation was being done. However, this estimation of regular rehabilitation at the clinic is not provided by Osakidetza-Basque Health Service, therefore the following cost-utility analysis was done just taking into account the current health service that PD has which is follow-up neurological consultations. This follow-up (consultations) had a cost of €661.27. Regarding the utilities, values from 0 to 1 have been obtained for the health states provided by the patients through the EQ-5D-5L quality of life survey. The intervention group had a mean health status of 0.2 at baseline, with some patients even having a health status worse than death (negative). After the intervention, it has gone on to have an average health status of 0.69. The control group has not suffered variation in their health status since these patients currently do not receive treatment.
The cost-effectiveness plane represented in Fig. 6 showed the difference in costs and results per patient. For a cost of €661.27 for the current treatment at the clinic, and a cost of €2,243.07 for telerehabilitation (vCare), given a threshold of €20,000 per QALY telerehabilitation was shown as a cost-effective alternative. The incremental cost and healthcare ratio QoL effects were the following: incremental cost in healthcare (23523.87), incremental effects (2.528), and incremental cost-effectiveness ratio in healthcare (9304.71).
Discussion
A pilot study was performed to evaluate the vCare system as a telerehabilitation tool, a monitorization of daily life, and a virtual coach in Parkinson’s disease (PD) patients. The vCare system performed daily life monitoring with presence and movement devices installed in the patient’s home. Patients performed a personalized telerehabilitation with a virtual coach system using an intelligent avatar that assists patients to perform their motor and cognitive rehabilitation by interacting with them and encouraging them to perform different activities that promote QoL. Findings from the present study confirmed that the PD vCare group of patients significantly increased their QoL, improved their general cognition, had fewer motor symptoms, and better daily life activities compared to the control group that did not use the vCare system, and showed a high adherence to all the outcomes measured.
Our study highlighted the significant improvement of the QoL dimensions in the vCare virtual coaching group after the intervention, obtaining better mobility, self-care, daily activities, and reduced pain. According to a case report study that performed telerehabilitation with a PD patient also showed an improvement in mobility and pain and discomfort, dimensions of QoL (Hoffmann et al. 2008). In this same line, a motor and cognitive telerehabilitation program performed with PD patients for a three-month period showed significant improvements in functional mobility (measured with the Two/2 Minute Walk Test), global cognitive function (measured with the MoCA) and quality of life (measured with the Mental Health Score of the SF-12 Health Survey) (Isernia et al. 2020). Another telerehabilitation study performed with a Nintendo Wii physical therapy program for PD patients showed a significant improvement in QoL total score and most of the specific domains measured with the 39-item Parkinson's Disease Questionnaire, after the intervention (Pedreira et al. 2013). A significant improvement was obtained in the daily activities of PD patients after the intervention However, we did not find significant differences in the UPDRS II evaluation post-intervention compared to pre-intervention, which corresponds to daily activities, before and after the intervention. In contrast with Pompeu et al. (2012), they found significant improvement in UPDRS II results after the intervention when they performed Wii-based motor and cognitive training (Pompeu et al. 2012). Other recent studies (Barksdale 2021; Hoffmann et al. 2008; Lee et al. 2015), also found a significant improvement in activities of daily living performing telerehabilitation, showing similar results to face-to-face rehabilitation. In this same line, in a study performed by van der Kolk et al. (2019), where PD patients performed indoor cycling for 30 to 45 minutes, at least three times per week, they found that aerobic exercises showed to improve motor symptoms reported in de UPDRS scale and to reduce the OFF state in PD patients (van der Kolk et al. 2019).
Regarding cognition, general cognition was also significantly improved after performing the vCare cognitive rehabilitation. Similar findings were reported in a study performed with Wii-based motor and cognitive training for PD patients, the cognitive improvement of those patients was maintained for 60 days after the end of training (Hoffmann et al. 2008; Pompeu et al. 2012). Maggio et al. (2018), also found cognitive improvement in attention, orientation, memory, fluency, language, and visuospatial, measured by the Mini-Mental State Examination and the Addenbrooke Cognitive Examination, after performing virtual reality cognitive training in PD patients (Maggio et al. 2018).
The monitorization of the PD symptoms was an important aspect of the follow-up during this pilot study. The STAT-ON device was used to collect data from different motor symptoms such as time in “ON” and “OFF”, dyskinesia, freezing of gait, or steps per day, better gait fluidity, quality of the patient’s walking (step length), fewer hours in OFF and more hours in ON was found. A relationship between time in ON status and dyskinesia as well as with UPDRS IV score was found, meaning a higher time spent in ON status was related to more dyskinesias (measured by the UPDRS IV). Dyskinesia could appear as a secondary effect of dopaminergic therapies, although our patients did not change their medication (Thanvi et al. 2007). Regarding the steps measured, higher levels of anxiety and depression were related to higher number of steps walked per day and more time walking (measured by the STAT-ON). However, we have not found any study that correlates anxiety and depression with more steps walked per day. In contrast, some studies have found a relationship between higher anxiety and more freezing of the gait (Martens et al. 2016). Our pilot study proved that this wearable device, the STAT-ON, provides objective information on the distribution and severity of PD motor symptoms in home environments (Rodríguez-Martín et al. 2022). According to Santos García et al. (2020), this device is more precise instrument than diaries for the monitorization of PD symptoms, and clinicians reported that they consider it a useful and secure tool for the follow-up of PD patients (Rodríguez-Martín et al. 2022; Santos García et al. 2020).
The adherence to the system was a fundamental aspect to take into account in our pilot study. This adherence was measured in the vCare system, adherence to the care plan, the motor and cognitive rehabilitation, and adherence to the devices. Similar findings were reported by Isernia et al. (2019, 2020) that evaluated the adherence to a motor and cognitive telerehabilitation program, showing high adherence and significant improvement in the motor and cognitive status of the patients in PD, stoke and multiple sclerosis (Isernia et al. 2019, 2020). According to Bianchini et al. (2022), adherence was found to be a good feasibility measure. They found that high adherence to the intervention was related to high feasibility in PD (Bianchini et al. 2022). In addition, home-based interventions with motivational apps and virtual coaching had been shown to promote prolonged adherence to motor rehabilitation programs for PD patients (van der Kolk et al. 2019). These coaches could be a key technology for empowering patients toward increasing their adherence to the care plan and improve their secondary prevention measures (Kyriazakos et al. 2020). According to van der Kolk et al. (2019), the possibility of exercising at home is an important facilitator for prolonged adherence.
Regarding usability and satisfaction, consistent with previous results in the living lab phase (Seregni et al. 2021), vCare patients in the pilot phase found that the telerehabilitation system was interesting, engaging, entertaining, challenging, and useful for improving impaired motor functions and making patients aware of their cognitive abilities.
Concerning the cost-utility analysis performed, telerehabilitation in Parkinson's patients was shown to be cost-effective compared to conventional rehabilitation, which recommends its implementation. Even if conventional rehabilitation was performed and if the improvement in QoL would not have been greater than through telerehabilitation, telerehabilitation would be the dominant alternative since it would be the most effective one and the least expensive alternative. In addition, it is important to highlight that vCare is not only a motor and cognitive telerehabilitation tool but also a virtual coach system that includes telerehabilitation, as well as an artificial intelligence and machine learning system that makes it possible for the rehabilitation and the avatar to adapt and personalize itself to each patient.
A virtual coach has the objective to optimize the user’s life through software that evaluates the performance of the patient and suggests actions, making a continuous adaptation of the coaching actions depending on the context to achieve specific goals. Weimann et al. (2022) defined the central aspects of Virtual coaching scenarios as an application system that contains user interfaces, data storage, and intelligence to process data and trigger and monitor coaching activities. This system must be placed in a natural context and interact with the user, and it must be initialized by a human coach or an existing knowledge base (Weimann et al. 2022). A humanoid avatar that serves as a virtual coach based on quality interactions with the patient could enable the continuity of care between hospital and home, focusing on a personalized coaching program and increasing adherence to rehabilitation (Tropea et al. 2019).
Our study has some limitations. First, this study had some technical problems during the first period of monitorization with some of the devices, and some data of a few patients got lost for the STAT-ON data and the presence of devices. Secondly, although the vCare avatar app made suggestions through a little red bell that appeared next to the avatar, it did not draw enough attention to the patients for them to realize that they had to click on the bell and see the message there, whether it was a reminder or a suggestion among others. This aspect should be rethought and shown to the patient differently. Thirdly, given the small sample size that tested the vCare solution, future studies should include a higher number of patients testing the vCare system. Although the small sample size (10 patients in the PD vCare group and 10 patients in the control group) and that two patients from the control group were lost at follow-up, we did find statistically significant differences in all the measures and high adherence to the system. Lastly, the control group did not perform any rehabilitation since the clinical care at the Basque health system includes regular clinical visits to the neurologists but no multidisciplinary rehabilitation for neurodegenerative diseases. The vCare system has been also implemented with patients with different pathologies at the other included pilot sited like heart failure, ischemic heart disease, and stroke, also finding positive results (Busnatu et al. 2022; Lăcraru et al. 2023).
Conclusions
In conclusion, this pilot study represents the first intervention through a virtual coach, cognitive and motor telerehabilitation, and daily-life monitoring in PD patients that includes a cost-utility study. Our study highlights the significant improvement in QoL, cognitive performance, motor symptoms, and activities of daily life in the vCare PD group compared to the control group. vCare patients found the vCare system was useful, novel, and stimulating and showed high adherence to the system, the care plan, rehabilitation, and devices. The vCare system was able to perform daily life monitoring with sensors installed in the patient’s house, adapt personalized rehabilitation, and promotes a healthy lifestyle. Telerehabilitation through the vCare system was a cost-effective alternative to conventional rehabilitation in PD patients. Therefore, as our results pointed out that telerehabilitation had a positive effect on the clinical status of PD patients. In addition to pharmacological treatment, non-pharmacological treatment should be offered to PD patients and included in the standard of care in the public health system.
Data availability
Data will be available upon request.
References
Barksdale H (2021) Outcomes Following Tele-rehabilitation in a Person with Parkinson Disease During the COVID-19 Pandemic: A Case Report. J Med Case Reports Case Series. https://doi.org/10.38207/jmcrcs/2021/0215224
Bianchini E, Onelli C, Morabito C, Alborghetti M, Rinaldi D, Anibaldi P, Marcolongo A, Salvetti M, Pontieri FE (2022) Feasibility, Safety, and Effectiveness of Telerehabilitation in Mild-to-Moderate Parkinson’s Disease. Front Neurol. https://doi.org/10.3389/fneur.2022.909197
Bickenbach J (2011) The world report on disability. Disability Soc. https://doi.org/10.1080/09687599.2011.589198
Brooke J (1986) Sus: a quick and dirty’usability. Usabil Evaluat Indust 189:189–194
Busnatu SS, Pană MA, Lăcraru AE, Jercălău CE, Paun N, Caprino M, Gand K, Schlieter H, Kyriazakos S, Andrei CL, Sinescu CJ (2022) Patient Perception When Transitioning from Classic to Remote Assisted Cardiac Rehabilitation. Diagnostics. https://doi.org/10.3390/diagnostics12040926
Del Pino R, Díez-Cirarda M, Ustarroz-Aguirre I, Gonzalez-Larragan S, Caprino M, Busnatu S, Gand K, Schlieter H, Gabilondo I, Gómez-Esteban JC (2022) Costs and effects of telerehabilitation in neurological and cardiological diseases: A systematic review. Front Med. https://doi.org/10.3389/fmed.2022.832229
Fahn S, Elton R, UPDRS Development Committee (1987) The Unified Parkinson’s Disease Rating Scale. Recent Developments in Parkinson’s Disease. 153–163
Gand K, Stark J, Schlieter H, Gißke C, Burwitz M (2021) Using Clinical Pathways To Virtual Coach Patients For Home Rehabilitation. ICIS 2021 Proceedings. https://aisel.aisnet.org/icis2021/is_health/is_health/7
Gillingham F, Donaldson M (1967) Schwab and England activities of daily living. In: Third Symposium of Parkinson’s Disease. E&S Livingstone, Edinburgh
Gißke C, Liu J, Gand K (2022) Applying Goal-Oriented Modelling for Machine Learning Based Rehabilitation Care. Stud Health Technol Inform. https://doi.org/10.3233/SHTI220471
Herlofson K, Larsen JP (2002) Measuring fatigue in patients with Parkinson’s disease-the Fatigue Severity Scale. Eur J Neurol. https://doi.org/10.1046/j.1468-1331.2002.00444.x
Hoehn MM, Yahr MD (1976) Parkinsonism: onset, progression, and mortality. Neurology 17:427–442
Hoffmann T, Russell T, Thompson L, Vincent A, Nelson M (2008) Using the Internet to assess activities of daily living and hand function in people with Parkinson's disease. NeuroRehabilitation. https://doi.org/10.3233/NRE-2008-23307
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiat. https://doi.org/10.1136/jnnp.55.3.181
Isernia S, Pagliari C, Jonsdottir J, Castiglioni C, Gindri P, Gramigna C, Palumbo G, Salza M, Molteni F, Baglio F (2019) Efficiency and Patient-Reported Outcome Measures From Clinic to Home: The Human Empowerment Aging and Disability Program for Digital-Health Rehabilitation. Front Neurol. https://doi.org/10.3389/fneur.2019.01206
Isernia S, Di Tella S, Pagliari C, Jonsdottir J, Castiglioni C, Gindri P, Salza M, Gramigna C, Palumbo G, Molteni F, Baglio F (2020) Effects of an Innovative Telerehabilitation Intervention for People with Parkinson’s Disease on Quality of Life, Motor, and Non-motor Abilities. Front Neurol. https://doi.org/10.3389/fneur.2020.00846
Kyriazakos S, Schlieter H, Gand K, Caprino M, Corbo M, Tropea P, Judica E, Sterpi I, Busnatu S, Philipp P, Rovira J, Martínez A, Lange M, Gabilondo I, Del Pino R, Carlos Gomez-Esteban JC, Pannese L, Bøttcher M, Lynggaard V (2020) A Novel Virtual Coaching System Based on Personalized Clinical Pathways for Rehabilitation of Older Adults—Requirements and Implementation Plan of the vCare Project. Front Digital Health. https://doi.org/10.3389/fdgth.2020.546562
Lăcraru AE, Busnatu Ștefan S, Pană MA, Olteanu G, Șerbănoiu L, Gand K, Schlieter H, Kyriazakos S, Ceban O, Andrei CL, Sinescu CJ (2023) Assessing the Efficacy of a Virtual Assistant in the Remote Cardiac Rehabilitation of Heart Failure and Ischemic Heart Disease Patients: Case-Control Study of Romanian Adult Patients. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph20053937
Lee NY, Lee DK, Song HS (2015) Effect of virtual reality dance exercise on the balance, activities of daily living, and depressive disorder status of Parkinson’s disease patients. J Phys Ther Sci. https://doi.org/10.1589/jpts.27.145
Maggio MG, De Cola MC, Latella D, Maresca G, Finocchiaro C, La Rosa G, Cimino V, Sorbera C, Bramanti P, De Luca R, Calabrò RS (2018) What About the Role of Virtual Reality in Parkinson Disease’s Cognitive Rehabilitation? Preliminary Findings From a Randomized Clinical Trial. J Geriatric Psychiat Neurol. https://doi.org/10.1177/0891988718807973
Martens KAE, Hall JM, Gilat M, Georgiades MJ, Walton CC, Lewis SJG (2016) Anxiety is associated with freezing of gait and attentional set-shifting in Parkinson’s disease: A new perspective for early intervention. Gait Posture. https://doi.org/10.1016/j.gaitpost.2016.07.182
Morgan C, Rolinski M, McNaney R, Jones B, Rochester L, Maetzler W, Craddock I, Whone AL (2020) Systematic Review Looking at the Use of Technology to Measure Free-Living Symptom and Activity Outcomes in Parkinson’s Disease in the Home or a Home-like Environment. J Parkinson’s Disease. https://doi.org/10.3233/JPD-191781
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699
Pedreira G, Prazeres A, Cruz D, Gomes I, Monteiro L, Melo A (2013) Virtual games and quality of life in Parkinson’s disease: A randomised controlled trial. Adv Parkinson’s Disease. https://doi.org/10.4236/apd.2013.24018
Peña J, Ibarretxe-Bilbao N, García-Gorostiaga I, Angeles Gomez-Beldarrain M, Díez-Cirarda M, Ojeda N (2014) Improving functional disability and cognition in Parkinson disease Randomized controlled trial. Neurology. https://doi.org/10.1212/WNL.0000000000001043
Pompeu JE, Mendes FA, Silva KG, Lobo AM, Oliveira TP, Zomignani AP, Piemonte MEP (2012) Effect of Nintendo WiiTMBased motor and cognitive training on activities of daily living in patients with Parkinson’s disease: A randomised clinical trial. Physiotherapy. https://doi.org/10.1016/j.physio.2012.06.004
Ramos-Goñi JM, Craig BM, Oppe M, Ramallo-Fariña Y, Pinto-Prades JL, Luo N, Rivero-Arias O (2018) Handling Data Quality Issues to Estimate the Spanish EQ-5D-5L Value Set Using a Hybrid Interval Regression Approach. Value Health. https://doi.org/10.1016/j.jval.2017.10.023
Rauschenberger M, Schrepp M, Perez-Cota M, Olschner S, Thomaschewski J (2013) Efficient Measurement of the User Experience of Interactive Products. How to use the User Experience Questionnaire (UEQ).Example: Spanish Language Version. Int J Interactive Multimed Artificial Intell. https://doi.org/10.9781/ijimai.2013.215
Rodríguez-Martín D, Cabestany J, Pérez-López C, Pie M, Calvet J, Samà A, Capra C, Català A, Rodríguez-Molinero A (2022) A New Paradigm in Parkinson’s Disease Evaluation With Wearable Medical Devices: A Review of STAT-ONTM. Front Neurol. https://doi.org/10.3389/fneur.2022.912343
Rosen MJ (1999) Telerehabilitation. NeuroRehabil. https://doi.org/10.3233/NRE-1999-12103
Santos García D, López Ariztegui N, Cubo E, Vinagre Aragón A, García-Ramos R, Borrué C et al (2020) Clinical utility of a personalized and long-term monitoring device for Parkinson’s disease in a real clinical practice setting: an expert opinion survey on STAT-ONTM. Neurologia. https://doi.org/10.1016/j.nrl.2020.10.013
Seregni A, Tricomi E, Tropea P, Del Pino R, Gómez-Esteban JC, Gabilondo I, Díez-Cirarda M, Schlieter H, Gand K, Corbo M (2021) Virtual Coaching for Rehabilitation: The Participatory Design Experience of the vCare Project. Front Public Health. https://doi.org/10.3389/fpubh.2021.748307
Thanvi B, Lo N, Robinson T (2007) Levodopa-induced dyskinesia in Parkinson’s disease: Clinical features, pathogenesis, prevention and treatment. Postgrad Med J. https://doi.org/10.1136/pgmj.2006.054759
Tropea P, Schlieter H, Sterpi I, Judica E, Gand K, Caprino M, Gabilondo I, Gomez-Esteban JC, Busnatu S, Sinescu C, Kyriazakos S, Anwar S, Corbo M (2019) Rehabilitation, the great absentee of virtual coaching in medical care: Scoping review. J Med Internet Res. https://doi.org/10.2196/12805
Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P (2018) Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. https://doi.org/10.1002/hec.3633
van der Kolk NM, de Vries NM, Kessels RPC, Joosten H, Zwinderman AH, Post B, Bloem BR (2019) Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: a double-blind, randomised controlled trial. Lancet Neurol. https://doi.org/10.1016/S1474-4422(19)30285-6
Warraich HJ, Califf RM, Krumholz HM (2018) The digital transformation of medicine can revitalize the patient-clinician relationship. npj Digital Med. https://doi.org/10.1038/s41746-018-0060-2
Weimann TG, Schlieter H, Brendel AB (2022) Virtual Coaches: Background, Theories, and Future Research Directions. Business Inform Syst Eng. https://doi.org/10.1007/s12599-022-00757-9
World Health Organization (2017) Violence and Injury Prevention Rehabilitation in health systems.
Acknowledgments
We want to thank all the members of the consortium who are involved in the vCare project. Thanks to Lucia Pannese and Vito Nitti from Imaginary (Italy), Alvaro Martinez from MYSPHERA (Spain), Daniel Rodriguez and Carlos Perez from sense4care (Spain), Luc Nicolas and Marc Lange from EHTEL (Belgium). Special thanks to all the clinicians who participated in this project from the Cruces University Hospital (Spain), Biocruces Bizkaia Health Research Institute (Spain), Department of Neurorehabilitation Sciences, Casa di Cura del Policlinico (Milan, Italy) and Faculty of Medicine, Carol Davila University of Medicine and Pharmacy (Bucharest, Romania).
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This project has received funding from the European Union’s Horizon 2020 Research and Innovation Program under grant agreement no: 769807.
Author information
Authors and Affiliations
Contributions
RDP contributed to the conception, design, and coordination of the study and interpretation of the results. RDP and AO contributed to the acquisition and analysis of data and the initial and final manuscript. RDP and JCGE supervised the project. All authors contributed to the critical revision of the manuscript and final version approval.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the regional Basque Clinical Research Ethics Committee (PS2021041).
Consent to participate
All participants gave written informed consent prior to their participation in the study, in accordance with the tenets of the Declaration of Helsinki.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 520 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Del Pino, R., de Echevarría, A.O., Díez-Cirarda, M. et al. Virtual coach and telerehabilitation for Parkinson´s disease patients: vCare system. J Public Health (Berl.) (2023). https://doi.org/10.1007/s10389-023-02082-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10389-023-02082-1