Abstract
Background
Nivolumab is recommended for patients with advanced esophageal squamous cell carcinoma (aESCC) refractory or intolerant to fluoropyrimidine- and platinum-based chemotherapy regardless of the tumor proportion score (TPS). However, the role of combined positive score (CPS) in predicting nivolumab efficacy remains unclear. We aimed to study whether TPS or CPS is a more suitable biomarker for predicting nivolumab efficacy in these patients.
Methods
We retrospectively collected data from patients with aESCC treated with fluoropyrimidines and platinum and subsequently received nivolumab monotherapy between January 1, 2014 and September 15, 2020. Next, we evaluated the efficiencies of TPS and CPS in predicting the clinical response to nivolumab using PD-L1 IHC 22C3 pharmDx assay.
Results
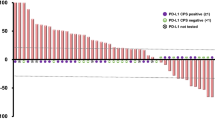
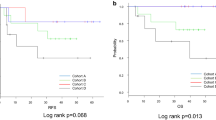
This study included 50 patients (CPS groups: ≥ 10/1–10/ < 1, n = 24/18/8, respectively; TPS groups, ≥ 10%/1%–10%/ < 1%, n = 17/8/25, respectively). The median progression-free survival was 3.2, 2.5, and 1.5 months in the ≥ 10, 1–10 [hazard ratio (HR) vs. CPS of ≥ 10 group, 1.01; p = 0.98; adjusted HR, 1.33; p = 0.56], and < 1 CPS groups (HR vs. CPS of ≥ 10 group, 3.44; p = 0.006; adjusted HR, 1.67; p = 0.41), respectively. For the patients with CPS of ≥ 10/1–10/ < 1 and TPS of ≥ 10%/1%–10%/ < 1%, the objective response rate was 30%/25%/0% and 36%/0%/19% and the disease control rate was 60%/50%/12% (p = 0.06) and 65%/40%/38% (p = 0.30), respectively.
Conclusions
This study suggests that a CPS of < 1 is not a strong predictor of efficacy but can predict the absence of response to nivolumab in patients with aESCC.


Similar content being viewed by others
Data availability
All data and materials used/generated for this study are available on request from the corresponding author.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Iizuka T, Kakegawa T, Ide H, et al. Phase II evaluation of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japanese esophageal oncology group trial. Jpn J Clin Oncol. 1992;22:172–6.
Bleiberg H, Conroy T, Paillot B, et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancer. Eur J Cancer. 1997;33:1216–20.
Hayashi K, Ando N, Watanabe H, et al. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan esophageal oncology group (JEOG) trial (JCOG9407). Jpn J Clin Oncol. 2001;31:419–23.
Hironaka S, Tsubosa Y, Mizusawa J, et al. Phase I/II trial of 2-weekly docetaxel combined with cisplatin plus fluorouracil in metastatic esophageal cancer (JCOG0807). Cancer Sci. 2014;105:1189–95.
Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–71.
Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–7.
Kojima T, Shah MA, Muro K, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–48.
Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–28.
Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50.
Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33.
Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–30.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35.
Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67.
Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–71.
Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13.
Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571–80.
Krigsfeld GS, Prince EA, Pratt J, et al. Analysis of real-world PD-L1 IHC 28–8 and 22C3 pharmDx assay utilisation, turnaround times and analytical concordance across multiple tumour types. J Clin Pathol. 2020;73:656–64.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
YM and HT conceptualized and designed the study. YM collected clinical data, drafted the manuscript, and performed the statistical analysis. HT performed the PD-L1 staining diagnosis of all tissue specimens. SK was responsible for this study and helped write the manuscript. WH performed PD-L1 staining and helped in pathological diagnosis. IO assisted in statistical analysis. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical statement and consent to participate
The institutional review board of Aichi Cancer Center Hospital, Japan, approved this study (R021076). The institutional review board also waived the requirement for informed consent because of the observational retrospective nature of this study, with an opt-out option indicated at the institution’s website.
Conflict of interest
YM received an honorarium from Takeda, Merck BioPharma, and Taiho. SK received an honorarium from Eli Lilly, Taiho, Ono, Bristol-Myers Squibb, Chugai, Bayer, Merck Serono, Daiichi Sankyo, and Esai as well as research funding from Taiho, Eli Lilly, MSD, Chugai, Nobelpharma, Ono, Daiichi Sankyo, and Yansen. TO received an honorarium from Ono, Bristol-Myers Squibb, Taiho, MSD, and Eli Lilly. TN received an honorarium from Eli Lilly. YN received an honorarium from Yakult Honsha, Taiho, Eli Lilly, Daiichi Sankyo, and AstraZeneca as well as research funding from Ono and Bristol-Myers Squibb. YN is also a member of the advisory board of Daiichi Sankyo. TM received an honorarium from Takeda, Chudai, Merck BioPharma, Taiho, Bayer, Eli Lilly, Yakult Honsha, Sanofi, Daiichi Sankyo, Ono, and Bristol-Myers Squibb as well as research funding from MSD, Daiichi Sankyo, Ono, and Novartis. HB received an honorarium from Ono, Eli Lilly, and Taiho as well as research funding from Ono. MT received an honorarium from EA Pharma, Otsuka Pharmaceutical Factory, and Bristol-Myers Squibb. KM received an honorarium from Ono, Chugai, Takeda, Taiho, Sanofi, Bristol-Myers Squibb, Eli Lilly, and Bayer; research funding from Solasia Pharma, Merck Serono, Daiichi Sankyo, Parexel International, Pfizer, MSD, Amgen, ONO, Astellas, Sanofi, Taiho, and Esai; and consulting fees from AstraZeneca, Ono, and Amgen. KM is also a member of the advisory boards of Ono, MSD, AstraZeneca, Daiichi Sankyo, and Solasia Pharma.
Consent to publish
All data and materials used for this study are available on request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10388_2022_978_MOESM2_ESM.docx
Supplementary file2 Supplementary Fig. 1 ROC curves showing the relation between CPS or TPS and the efficacy outcomes: a response, b disease control, and c disease progression. ROC receiver operating characteristic, CPS combined positive score, TPS tumor proportion score, AUC areas under the curves (DOCX 236 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsubara, Y., Toriyama, K., Kadowaki, S. et al. The impact of combined PD-L1 positive score on clinical response to nivolumab in patients with advanced esophageal squamous cell carcinoma. Esophagus 20, 524–532 (2023). https://doi.org/10.1007/s10388-022-00978-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-022-00978-7