Abstract
Purpose
To assess the efficacy and safety of filtration surgery using the EX-PRESS glaucoma filtration device in patients with normal-tension glaucoma (NTG).
Study design
Prospective, single-arm, multicenter interventional case series.
Methods
Eyes with NTG underwent EX-PRESS implantation with or without cataract surgery. The efficacy and safety were assessed at 1 day; 1 and 2 weeks; and 1, 3, 6, and 12 months after surgery. The main outcome measure was reduction in intraocular pressure (IOP) from baseline at 3, 6, and 12 months after surgery. Safety assessments included adverse event incidence, postoperative inflammation, and corneal endothelial cell density.
Results
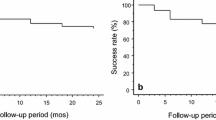
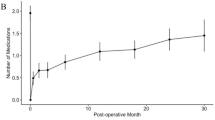
Thirty-two Japanese patients (37 eyes) with NTG were enrolled. The mean IOP decreased from 14.8 ± 2.3 mmHg at baseline to 10.0 ± 3.1 mmHg at 12 months after surgery (mean reduction 4.9 ± 4.2 mmHg [31.1%]; P < .0001). IOP-lowering medication use decreased from a mean of 3.3 medications per eye before surgery to 0.1 medications per eye at 12 months after surgery. IOP reductions > 20% were achieved by 61.5% of the eyes at 12 months. Adverse events were typical for filtration procedures, and none was deemed device-related. Postoperative inflammation was mild and self-limiting. The mean corneal endothelial cell density had decreased by 3.3% at 12 months after surgery.
Conclusion
The EX-PRESS glaucoma filtration device is safe and effective for filtration surgery in patients with NTG, providing mean IOP reduction consistent with recommendations based on the Collaborative NTG Study.




Similar content being viewed by others
References
Schulzer M. Intraocular pressure reduction in normal-tension glaucoma patients: the Normal Tension Glaucoma Study Group. Ophthalmology. 1992;99:1468–70.
Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505.
Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97.
CNTGS: Collaborative Normal Tension Glaucoma Study (1998). In: Clinical Trials in Glaucoma. American Academy of Ophthalmology. 2017. http://eyewiki.aao.org/Clinical_Trials_in_Glaucoma#3._CNTGS:_Collaborative_Normal_Tension_Glaucoma_Study_.281998.29. Accessed 2 Jan 2018.
Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC, Tube Versus Trabeculectomy Study Group. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–14 e1.
Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL, Tube Versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153:789–803 e2.
Membrey WL, Poinoosawmy DP, Bunce C, Hitchings RA. Glaucoma surgery with or without adjunctive antiproliferatives in normal tension glaucoma: 1 intraocular pressure control and complications. Br J Ophthalmol. 2000;84:586–90.
Bovee CE, Pasquale LR. Evolving surgical interventions in the treatment of glaucoma. Semin Ophthalmol. 2017;32:91–5.
EXPRESS Mini Glaucoma Shunt. In: Devices@FDA. United States Food and Drug Administration. https://www.accessdata.fda.gov/scrIpts/cdrh/devicesatfda/index.cfm?db=pmn&id=K030350. Accessed 2 Jan 2018.
Traverso CE, De Feo F, Messas-Kaplan A, Denis P, Levartovsky S, Sellem E, et al. Long term effect on IOP of a stainless steel glaucoma drainage implant (Ex-PRESS) in combined surgery with phacoemulsification. Br J Ophthalmol. 2005;89:425–9.
Dahan E, Carmichael TR. Implantation of a miniature glaucoma device under a scleral flap. J Glaucoma. 2005;14:98–102.
Maris PJ Jr, Ishida K, Netland PA. Comparison of trabeculectomy with Ex-PRESS miniature glaucoma device implanted under scleral flap. J Glaucoma. 2007;16:14–9.
de Jong L, Lafuma A, Aguade AS, Berdeaux G. Five-year extension of a clinical trial comparing the EX-PRESS glaucoma filtration device and trabeculectomy in primary open-angle glaucoma. Clin Ophthalmol. 2011;5:527–33.
Ishida K, Moroto N, Murata K, Yamamoto T. Effect of glaucoma implant surgery on intraocular pressure reduction, flare count, anterior chamber depth, and corneal endothelium in primary open-angle glaucoma. Jpn J Ophthalmol. 2017;61:334–46.
Iijima Y. Ethical guidelines for clinical studies: overview [in Japanese]. Rinsho Shinkei. 2011;51:830–3.
Schultz SK, Iverson SM, Shi W, Greenfield DS. Safety and efficacy of achieving single-digit intraocular pressure targets with filtration surgery in eyes with progressive normal-tension glaucoma. J Glaucoma. 2016;25:217–22.
Iverson SM, Schultz SK, Shi W, Feuer WJ, Greenfield DS. Effectiveness of single-digit IOP targets on decreasing global and localized visual field progression after filtration surgery in eyes with progressive normal-tension glaucoma. J Glaucoma. 2016;25:408–14.
Jayaram H, Strouthidis NG, Kamal DS. Trabeculectomy for normal tension glaucoma: outcomes using the Moorfields Safer Surgery technique. Br J Ophthalmol. 2016;100:332–8.
Chan JE, Netland PA. EX-PRESS glaucoma filtration device: efficacy, safety, and predictability. Med Devices (Auckl). 2015;8:381–8.
Shaarawy T, Goldberg I, Fechtner R. EX-PRESS glaucoma filtration device: review of clinical experience and comparison with trabeculectomy. Surv Ophthalmol. 2015;60:327–45.
Miyamoto D, Sakaue Y, Togano T. Corneal endothelial cell change after filtration surgery using a micro-tube shunt (Ex-PRESS) [in Japanese]. J Eye. 2016;33:1645–50.
Kim MS, Kim KN, Kim CS. Changes in corneal endothelial cell after Ahmed glaucoma valve implantation and trabeculectomy: 1-year follow-up. Korean J Ophthalmol. 2016;30:416–25.
Casini G, Loiudice P, Pellegrini M, Sframeli AT, Martinelli P, Passani A, et al. Trabeculectomy versus EX-PRESS shunt versus Ahmed valve implant: short-term effects on corneal endothelial cells. Am J Ophthalmol. 2015;160:1185–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
M. Aihara, Research fee (Alcon), Support for Study design/Manuscript preparation/ Statistical analysis (Alcon), Financial support (Alcon Pharma, AMO, CREWT medical systems, Glaukos, Kowa Pharmaceutical, Nitten, Novartis, Ono, Otsuka, Pfizer, Santen, Senju, TOMEY, Wakamoto), Consultant fee (Alcon Pharma, CREWT medical systems, Glaukos, HOYA, InnFocus, Iridex, Kowa, Ono, Otsuka, Pfizer, Santen, Senju, Wakamoto), Lecture fee (Alcon Pharma, AMO Japan, Canon, Carl Zeiss Meditec, CREWT medical systems, Glaukos, HOYA, InnFocus, Iridex, Ivantis, Kowa Pharmaceutical, NIDEK, Nitten, Novartis, Ono, Otsuka, Pfizer, Santen, Senju, TOMEY, Alcon), Travel fee (Alcon Pharma, AMO Japan, Canon, Carl Zeiss Meditec, CREWT medical systems, Glaukos, HOYA, InnFocus, Iridex, Ivantis, Kowa Pharmaceutical, NIDEK, Nitten, Novartis, Ono, Otsuka, Pfizer, Santen, Senju, TOMEY) Grant (Alcon); Y. Kuwayama, Research fee (Alcon), Lecture fee (Alcon Pharma, Glaukos Japan, Kowa, Otsuka, Pfizer, Santen, Senju), Consultant fee (Kowa, Otsuka, Pfizer, Santen, Senju, Aerie Pharmaceuticals, Wakamoto); K. Miyata, Research fee (Alcon), Lecture Fee (Alcon, AMO, HOYA, Senju, Santen, Wakamoto, Kowa, Otsuka, Chuosangio, TOMEY, Staar), Grant (AMO, HOYA, Senju, Shionogi, Santen, Novartis, Wakamoto, Kissei, Sucampo Pharma); S. Ohtani, Research fee (Alcon) ; R. Ideta, Lecture fee (Alcon Pharma, Otsuka, Pfizer, Santen, Wakamoto); Y. Hashimoto, None; N. Sasaki, Employee (Alcon); S. Shirato, Research fee (Alcon), Lecture fee (Alcon).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding author: Makoto Aihara
About this article
Cite this article
Aihara, M., Kuwayama, Y., Miyata, K. et al. Twelve-month efficacy and safety of glaucoma filtration device for surgery in patients with normal-tension glaucoma. Jpn J Ophthalmol 63, 402–409 (2019). https://doi.org/10.1007/s10384-019-00682-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-019-00682-7