Summary
Background
Colorectal cancer (CRC) is the fourth most common cause of death worldwide. Approximately 30 % of all CRC occurs in the rectum. Improvements in survival rates were achieved thanks to multimodal therapy, combining surgery and chemoradiation. Nevertheless, the prognosis of patients suffering from rectal cancer (RC) remains poor. Programmed cell death protein 1 (PD-1) and its ligand programmed death ligand 1 (PD-L1) regulate tumor immune response. The aim of this study was to analyze the expression of PD-L1 in RC pre- and post-neoadjuvant therapy and evaluate PD-L1 as a biomarker and potential target for therapy.
Methods
In all, 29 patients with RC treated at the Medical University Vienna who received preoperative chemoradiation were retrospectively enrolled in this study. Expression of PD-L1 was investigated by immunohistochemistry with two different anti-PD-L1 antibodies.
Results
No PD-L1 expression on cancer cells could be observed in all 29 cases in the specimens before chemoradiation as well as in the surgical specimens after neoadjuvant therapy. In one of the two staining methods performed, five (17.24 %) post-chemoradiation cases showed faint lymphohistiocytic staining.
Conclusion
No expression of PD-L1 in RC cells before and after chemoradiation was found in our collective of 29 patients. Further investigations to evaluate the role of PD-L1 as a potential therapeutic target in RC are urgently needed.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-related death worldwide [1]. The standard treatment of locally advanced rectal cancer (RC) is neoadjuvant chemoradiation (NCR) followed by surgical resection [2]. Despite the usage of multimodal neoadjuvant therapy and improved survival rates, the prognosis of patients with RC remains unsatisfying. After neoadjuvant treatment combined with surgical resection, histopathological response rates differ enormously [3]. In recent years, significant insights into the interactions between the immune system and cancer cells have been gained. Recently, programmed death ligand 1 (PD-L1) raised scientific interest, as the first clinical studies with PD-L1 inhibitors promised encouraging results in several tumor types [4–7].
The binding of PD-L1 to its receptor programmed cell death protein 1 (PD-1) plays a major role in the interaction between cancer and the immune system. PD-1 belongs to the CD28/CTLA-4 immunoglobulin family and is expressed on activated T and B cells, monocytes, and tumor-infiltrating lymphocytes (TILs). PD-L1 can be found on resting T cells, B cells, macrophages, and vascular endothelial cells [8, 9]. The PD-1–PD-L1 interaction plays a key role in maintaining self-tolerance to protect against severe self-damage while the immune system is activated because of infections. Tumor cells can overexpress PD-L1, which binds to PD-1 on T cells and inhibits their activation. As a result, the tumor escapes surveillance by the immune system [10–12].
PD-L1 has been reported to be expressed in a number of malignancies, especially by glioblastoma, non-small-cell lung cancer, melanoma, renal cell carcinoma, and esophageal cancer [13–17]. Although PD-L1 is present in the cytoplasm and on the plasma membrane of tumor cells, not every type of cancer expresses PD-L1 [8, 18]. Clinical trials report promising data, when investigating PD-1 and/or PD-L1 blockade using monoclonal antibodies. Nevertheless, only a small number of patients benefit from inhibiting PD-1 or PD-L1 [7, 19, 20].
Recently, a difference in expression of PD-L1 in post-neoadjuvant therapy tumor tissue compared with pre-neoadjuvant therapy tumor tissue was found for various cancers [21–23]. To date, the expression of PD-L1 in neoadjuvant-treated RC has not been investigated intensively. In this single-center study, we examined the effect of neoadjuvant treatment on the expression of PD-L1 of locally advanced RC.
Material and methods
Patients
In this retrospective analysis all patients underwent surgery for RC at the Department of Surgery, Medical University of Vienna, between 1 January 2012 and 31 December 2013. The study was approved by the Ethics Committee of the Medical University of Vienna, Austria, according to the Declaration of Helsinki. The study population consisted of patients who underwent total mesorectal excision (TME) after receiving capecitabine-based neoadjuvant chemoradiation (NCR) at the University Hospital Vienna, Austria (Fig. 1). Chemoradiation was delivered as long-course radiation (LCRT) with 50.4 Gy (1.8 Gy in 28 fractions). Patients presenting with distant metastatic disease at the time of diagnosis were excluded from the analysis.
PD-L1 expression in the context of NCR was investigated on pre-NCR biopsies and post-NCR surgical specimens. The clinicopathological factors selected and analyzed were age, gender, TNM staging according to the Union Contre le Cancer (UICC) 7th edition TNM classification, and the response rate to NCR [24].
Assessment of response to NCR
Post-NCR specimens were analyzed for tumor response to neoadjuvant treatment. Response to neoadjuvant chemoradiation is reported as the ratio of viable tumor tissue and fibrosis. Tumor response rates were analyzed according to Dworak et al. [25]. Response was graded as: 0 – no regression; 1 – dominant tumor mass with obvious fibrosis and/or vasculopathy; 2 – dominantly fibrotic changes with few tumor cells or groups; 3 – very few (difficult to find microscopically) tumor cells in fibrotic tissue with or without mucous substance; 4 – no tumor cells, only fibrotic mass (total regression or response).
Immunohistochemistry
Immunohistochemistry (IHC) was performed on paraffin-embedded specimens fixed in 4 % buffered formalin, using 3‑µm-thick histological sections. For PD-L1 expression in pre- and post-NCR tissue, two different staining methods were used. The first staining method was performed on a Ventana® BenchMark ULTRA (Roche, Tucson, AZ), automated IHC slide staining system, using the universal DAB detection kit with cell conditioning 1 (CC1) buffer of pH 8 and the PD-L1 (E1L3N®) XP® rabbit mAb (Cell Signalling Technology, Danvers, MA).
The second staining method was performed manually, using the antibody 5H1 (monoclonal antibody of human B7H1, Dr. Lieping Chen’s lab, isotype: mouse IGg1) as described previously [18].
PD-L1 expression was scored as positive or negative with semiquantitative estimation of percentage of positive tumor and stroma cells. Slides stained according to the first staining method were independently reviewed and scored by two observers (M.L. and J.G.). All slides stained according to the second staining method were reviewed and scored by M.B.
Statistical analysis
Correlations between clinicopathological factors and expression of PD-L1 were analyzed with the χ2 test or Fisher’s exact test. Statistical analyses were performed, using SPSS 21.0 (IBM Corp.©, SPSS©) software.
Results
The final study population consisted of 29 patients (82.76 % males and 17.24 % females), who were eligible for further investigations. Overall median age at the time of surgery was 65 years (23–85 years). The majority of patients (68.97 %) were radiologically in advanced UICC stages at the time of diagnosis (Table 1).
All study patients received LCRT and completed NCR.
The histopathological results of the surgical specimens showed three patients (10.34 %) with complete response (CR, grade 4), 22 patients (75.86 %) with partial response (PR, grades 3–1), and four patients (13.8 %) with no response to NCR at all (nonresponders, NR; details in Table 1).
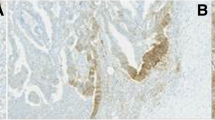
For both immunohistochemical staining methods employed, no expression of PD-L1 on neoplastic cells could be detected in all pre-NCR (29 cases, 100 %), as well as in all post-NCR cases (29 cases, 100 %). In five post-NCR cases (17.24 %) stained according to the first staining method, faint lymphohistiocytic staining (intensity score 1) of TILs could be observed (Fig. 2). Two of those five cases showed CR, two PR, and one was NR (Table 2).
a–c Representative hematoxylin–eosin (H&E)-stained rectal cancer (RC) specimens after neoadjuvant chemoradiation (NCR) (magnification ×400). d, e Corresponding immunohistochemical PD-L1 stainings, showing PD-L1-positive immune cells in the tumor stroma of RC after NCR (magnification ×400). f RC showing no expression of PD-L1 in immunohistochemical PD-L1 staining after NCR (magnification ×400)
Discussion
Even after adequate surgery, still around 30 % of patients with RC suffer from metastatic disease within 5 years of surgery. Currently established adjuvant chemotherapeutic agents have limited potential to prevent recurrence [2, 3]. Alternative treatment options are currently tested in clinical trials to improve long-term outcome [26].
Immunotherapy offers new opportunities in cancer treatment. Anti-PD-L1 antibodies showed encouraging results in different cancer patients, especially melanoma and recently non-small-lung cancer [19, 20]. In comparison with standard treatment, using chemo- and/or radiotherapy, immunotherapy is relatively safe, effective, and has low-grade side effects. Consecutively, this treatment modality gained high interest in CRC, too [27, 28].
Recently, Droeser et al. analyzed nearly 1,500 CRC samples, showing more than 30 % PD-L1 expression in untreated tumor tissue [29]. In contrast to other cancer types [21, 22], limited data are published on PD-L1 expression in RC and a potential impact of neoadjuvant therapy on PD-L1 expression. Therefore, the aim of this study was to investigate the expression of PD-L1 in a cohort of 29 neoadjuvant treated-patients suffering from locally advanced CRC.
Unfortunately, we could not detect PD-L1 expression neither in pre-NCR nor in post-NCR tumor tissues. Nevertheless, we observed expression of PD-L1 on TILs, which are considered to represent the immune response of the host to a malignant tumor, in five post-NCR cases. Expression of PD-L1 in 17.24 % of post-NCR TILs might implicate that the PD-L1/TAM (tumor-associated macrophages) and PD-1/TIL pathway is activated.
The significantly higher rate of PD-L1 expression in the study by Droeser et al. might be explained by a different study population combining patients with colon cancer and RC. We know from Xiao and Freedman that a microsatellite instable (MSI) subset of CRC might be a promising target for checkpoint immunotherapy [30]. Although Devaraj et al. reported that MSI in RC is rare, to our knowledge, little is known about the expression of PD-L1 in an MSI subset of RC to date [31].
Therefore, even though Droeser et al. reported more than 30 % PD-L1 expression in CRC tumor tissue, further investigations in developing PD-L1/PD-1 based immunotherapy should focus on the cell-type-specific analysis of the tumor surrounding the stroma. As mentioned, an MSI subset of CRC might be a promising target for checkpoint immunotherapy [30]. Based on these results, the MSI subset might be a potential therapeutic target in RC as well. Therefore, the role of PD-L1 as biomarker or target in immunotherapy in neoadjuvant-treated RC needs further investigation.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Derbel O, Wang Q, Desseigne F, Rivoire M, Meeus P, Peyrat P, et al. Impact of KRAS, BRAF and PI3KCA mutations in rectal carcinomas treated with neoadjuvant radiochemotherapy and surgery. BMC Cancer. 2013;13:200.
Swaika A, Hammond WA, Joseph RW. Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy. Mol Immunol. 2015;67(2 Pt A):4–17.
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–9.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T‑cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64.
Ramsay AG. Immune checkpoint blockade immunotherapy to activate anti-tumour T‑cell immunity. Br J Haematol. 2013;162(3):313–25.
Li J, Jie HB, Lei Y, Gildener-Leapman N, Trivedi S, Green T, et al. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res. 2015;75(3):508–18.
Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116.
Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104(10):2084–91.
Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94(1):107–16.
Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–53.
Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63(21):7462–7.
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37.
Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65.
Lim SH, Hong M, Ahn S, Choi YL, Kim KM, Oh D, et al. Changes in tumour expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancer. Eur J Cancer. 2016;52:1–9.
Sheng J, Fang W, Yu J, Chen N, Zhan J, Ma Y, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep. 2016;6:20090.
Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015;3(4):326–32.
Sobin LH, Wittekind C. International Union Against Cancer (UICC) TNM classification of malignant tumors, 7th ed. 2009.
Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12(1):19–23.
Gulbake A, Jain A, Jain A, Jain A, Jain SK. Insight to drug delivery aspects for colorectal cancer. World J Gastroenterol. 2016;22(2):582–99.
Vennepureddy A, Thumallapally N, Motilal Nehru V, Atallah JP, Terjanian T. Novel drugs and combination therapies for the treatment of metastatic melanoma. J Clin Med Res. 2016;8(2):63–75.
Zhou C, Yao LD. Strategies to improve outcomes of patients with EGRF-mutant non-small cell lung cancer: review of the literature. J Thorac Oncol. 2016;11(2):174–86.
Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013;49(9):2233–42.
Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 2015;5(1):16–8.
Devaraj B, Lee A, Cabrera BL, Miyai K, Luo L, Ramamoorthy S, et al. Relationship of EMAST and microsatellite instability among patients with rectal cancer. J Gastrointest Surg. 2010;14(10):1521–8.
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G.Jomrich, G.R.Silberhumer, B.Marian, A.Beer, L.Müllauer declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jomrich, G., Silberhumer, G.R., Marian, B. et al. Programmed death-ligand 1 expression in rectal cancer. Eur Surg 48, 352–356 (2016). https://doi.org/10.1007/s10353-016-0447-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-016-0447-8