Abstract
Knowledge of the relationships between food habits and habitat is crucial for the assessment of habitat quality for birds. The present study investigated the diet and reproductive success of Little Bitterns Ixobrychus minutus nesting on cyprinid fish ponds, an important breeding habitat of this species in central and eastern Europe. Being subject to different management practices, fish ponds provide food resources of uneven availability for this small heron. Prey items regurgitated by nestlings were examined, and breeding success was estimated on monoculture ponds stocked either with small fish (of a size suitable for feeding nestlings) or large fish (unavailable to Little Bitterns and adversely affecting their non-fish prey), on abandoned ponds dominated by small fish but with large fish also present, and on angling ponds dominated by large sport fish but harbouring significant numbers of small fish as well. A total of 1356 prey items from 78 broods were identified. Although Little Bitterns exhibited dietary flexibility in response to the contrasting availability of prey on their nesting ponds, the bulk of the nestlings’ diet consisted of fish. The size of fish brought to the nest increased significantly with brood age, showing that parents adjusted the prey size to the gape constraints of their young. The chick production determined for 73 broods did not differ with respect to pond management, but the dietary composition indicated that to compensate for food shortages, birds nesting on ponds containing mainly large fish made foraging flights to food-richer ponds. The abundance of small-sized fish prey may be a factor limiting the breeding success of small- and medium-sized predatory waterbirds and should be taken into consideration in management strategies of habitats dominated by fish.
Zusammenfassung
Nahrungsökologie und Reproduktionserfolg der Zwergdommel Ixobrychus minutus in unterschiedlich bewirtschafteten Teichbiotopen
Die Beziehungen zwischen Ernährungsgewohnheiten und Lebensraum bei Vögeln zu kennen, ist für die Beurteilung der Qualität eines Habitats von entscheidender Bedeutung. In der vorliegenden Studie wurden die Ernährung und der Fortpflanzungserfolg von Zwergdommeln Ixobrychus minutus untersucht, die in Karpfenteichen, einem wichtigen Bruthabitat dieser Art in Mittel- und Osteuropa, nisten. Da Fischteiche unterschiedlich bewirtschaftet werden, bieten sie dieser kleinen Reiherart unterschiedlich verfügbare Nahrungsquellen. Die von den Nestlingen erbrochenen Reste von Beutetieren wurden untersucht und der Bruterfolg bei Teichen mit Monokulturen eingeschätzt, die entweder mit kleinen Fischen in für die Jungvögel geeigneter Größe oder mit großen Fischen bestückt waren, die für die Zwergdommel zu groß waren und somit einen Einfluss darauf hatten, welche Art Nahrung außer Fischen die Vögel zu sich nahmen. Ferner wurden aufgelassene Teiche mit überwiegend kleinen, aber auch einigen großen Fischen berücksichtigt wie auch Angelteiche, in denen große Sportfischarten dominierten, aber auch eine beträchtliche Anzahl kleiner Fische vorhanden war. Insgesamt wurden 1356 Beutereste aus 78 Bruten berücksichtigt. Obwohl die Zwergdommeln ihre Ernährung flexibel an die unterschiedliche Verfügbarkeit von Beutetieren in ihren Nistteichen anpassten, bestand der Großteil der Nahrung der Nestlinge aus Fisch. Die Größe der Fische, die zum Nest gebracht wurden, nahm mit dem Alter der Brut signifikant zu, was zeigt, dass die Eltern die Größe der Beute an die Größe der Schnabelöffnung ihrer Jungen anpassen. Die Produktion von Jungen, wie sie für 73 Bruten ermittelt wurde, unterschied sich nicht in Hinblick auf die Teichbewirtschaftung, aber die Nahrungszusammensetzung deutete darauf hin, dass die Vögel, die in Teichen mit überwiegend großen Fischen nisteten, Futterflüge zu nahrungsreicheren Teichen unternahmen, um einen möglichen Nahrungsmangel auszugleichen. Die Menge an kleinen Fischen in einem Teich kann ein Faktor sein, der den Bruterfolg kleiner und mittelgroßer räuberischer Wasservögel einschränkt und sollte deshalb bei Überlegungen zum Bewirtschaftungsmanagement von Fischteichen berücksichtigt werden.
Similar content being viewed by others
Introduction
The recent anthropogenic degradation of aquatic and wetland ecosystems has reduced the availability of natural breeding habitats of many bird species, resulting in strong declines in their populations (Dudgeon et al. 2006; Ma et al. 2010). Waterbirds often colonise man-made habitats, so effective species protection strategies require an understanding of both species’ breeding requirements and the distinctive features of such habitats. Since food resources are a factor limiting breeding performance in many birds (Martin 1987), the first step is to acquire insight into the links between species’ feeding habits and the resources that a particular habitat offers. Open fish ponds, often constructed to the detriment of natural wetlands, provide vast and attractive breeding areas for birds (Cheng et al. 2022). However, fish culture practices, such as specific fish stocking strategies, determine the abundance and diversity of prey for waterbirds (Horváth et al. 1992; Haas et al. 2007; Kloskowski 2011, 2012). The principal aim of aquaculture is the rapid production of large marketable fish, but this constrains suitable foraging and breeding habitats of many waterbirds (Kloskowski et al. 2010; Kloskowski 2012). Fish, though typically more profitable prey than invertebrates or amphibian larvae (Jackson 2003), can interact with birds as competitors capable of strong indirect effects (Kloskowski 2011; Nummi et al. 2016; Maceda-Veiga et al. 2017). Hence, although piscivorous birds appear to benefit from the presence of fish, the habitat selection and breeding success of generalist avian predators can be adversely affected by fish, especially when the latter attain a size protecting them from avian predation (Kloskowski 2012; see also Eriksson 1986).
The Little Bittern Ixobrychus minutus (L., 1766), the smallest member of the family Ardeidae in Europe (Cramp and Simmons 1977), is one of the least studied herons on this continent (Voisin 1991; Pardo-Cervera et al. 2010). In response to the decline of natural wetlands in many parts of its range, the species has shifted to human-modified habitats (Cramp and Simmons 1977; Keller et al. 2020). The majority of the central and eastern European population breed on open fish ponds, traditionally used for cyprinid culture (Švažas et al. 2000; Tomiałojć and Stawarczyk 2003). Like most herons, the Little Bittern is considered an opportunistic predator (Voisin 1991), but its small body size (adult body mass 120–140 g, bill length 44–53 mm, body mass at hatching 9–12 g; Cramp and Simmons 1977; Pardo-Cervera et al. 2010) imposes a significant constraint on prey size selection. Research to date shows that it feeds mainly on fish, but amphibians and macroinvertebrates also make up a considerable proportion of its food. Nevertheless, its dietary composition has not been directly related to prey abundance or reproductive success (Vasvari 1929; Moltoni 1948; Holmes and Hatchwell 1991; Melikyan 2008; Kayser 2010; Pardo-Cervera et al. 2010; Flis and Gwiazda 2018; but see Trnka 2020).
In species whose chicks are entirely dependent on parental feeding, a critical challenge to the parent birds is to meet the time-progressive nutritional and energy demands of their young. Parents can supply prey of increasing size as the chicks grow (e.g. Stienen et al. 2000; Hampl et al. 2005) or increase the frequency of nest visits (Bryan et al. 1995; Campos and Lekuona 1997). In fact, many studies show a positive correlation between chick age and the size of delivered prey items both in herons (e.g. Moser 1986; Campos and Lekuona 1997) and in other waterbirds (e.g. Kloskowski 2004; Fernández Ajó et al. 2011). Other research, however, indicates that herons, which feed their offspring by regurgitation, largely avoid the necessity of adjusting prey size by provisioning partially digested food (Owen and Phillips 1956; Kushlan 1978; Kim and Yoo 2012).
The aim of this study was to investigate the food habits and food-related breeding success of Little Bitterns on managed ponds varying in prey availability. We predicted that the dietary composition of nestlings would differ between ponds, depending on the size structure of their fish populations. We also predicted that pond management practices would influence the species’ breeding success, this being greater on ponds containing small fish and also rich in non-fish prey, and lower on ponds with fish size structures biased toward sizes unsuitable for Little Bitterns. Moreover, we aimed to track parental feeding strategies, such as prey size adjustment to nestling size and potential changes in the dietary contribution of fish, the dominant food component, with the growth of the young birds.
Study area and methods
Study system
The field work was conducted during the 2014–2017 breeding seasons on ponds aggregated in four irregular clusters (water area 15–185 ha) scattered at distances of 10–80 km from each other around the city of Lublin, eastern Poland (51° 00′–51° 27′ N, 22° 15′–23° 22′ E). Within the clusters, the nearest adjacent ponds were often separated only by levees about 8–10 m wide (Fig. 1). The lake-like ponds were inundated with water from precipitation or from adjacent rivers. The surface area of the ponds occupied by Little Bitterns ranged from 0.2 to 26.9 ha. The ponds varied in size and in emergent vegetation cover, but all of them had a similar morphometry, such as the pond profile or mean depth (about 0.9–1.3 m).
To assess the relative abundance of macroinvertebrates, small fish and amphibians in the ponds, submerged activity traps were used. These were made from 1-L plastic cylinders with funnels 100 mm wide at the large end and 23 mm wide at the narrow end (Nieoczym and Kloskowski 2015; see also Hyvönen and Nummi 2000). Ten such traps were set in each sampled pond for 48 h between 17 June and 5 July, the peak hatching period of the Little Bitterns in the study area (median first-egg hatching date: 30 June). The traps were deployed horizontally at least 10 m apart, around the entire perimeter of the pond, at the interface between the emergent vegetation and the open water (excluding shoreline areas devoid of vegetation), where Little Bitterns were frequently observed to forage. Data were collected between 2003 and 2014, so most of the ponds (except the abandoned ponds where trapping was carried out exclusively in 2014) had been sampled in the years preceding the research on the diet and breeding success of Little Bitterns. However, assuming that the fish stocking management practices were the same (see below), prey availability was highly consistent from one year to another in the ponds because the abundance of macroinvertebrates and amphibians in them was predominantly contingent on the fish status (fish size structure and density) of the pond (Kloskowski 2011; Nieoczym and Kloskowski 2015). Data on numbers and size structures of the stocked fish, along with information on the abundance of other, wild-grown species in the ponds, were provided by the local fisheries (Table 1). The body size at which a fish becomes safe from the gape-limited Little Bittern varies, depending on the fish body depth and the swallowing capacities of the chicks. In our study, 95% of the fish consumed by nestlings were estimated to be < 90 mm of the total length (longitudo totalis, LT; see Results), the value approximately defining the swallowing constraints of 7–10-day-old hatchlings.
The study ponds were assigned to four types, irregularly interspersed within the pond aggregations, including semi-extensive monoculture Carp ponds (cf. Horváth et al. 1992) drained each autumn and replenished with water in spring. (1) Monoculture ponds with young fish (hereafter ‘monoculture small-fish ponds’): They were stocked with Common Carp Cyprinus carpio fry at an individual body weight of 1–3 mg throughout May, and within a few weeks, the young fish attained a size suitable for consumption by birds (individual weight of about 3–7 g at a LT of 40–70 mm by the end of June). Given the low biomass density and the weak trophic impact of the young Carp, abundant macroinvertebrate and amphibian resources were available in these ponds (Table 1). (2) Monoculture ponds containing large, 1-year-old or 2-year-old Common Carp (‘monoculture large-fish ponds’), stocked almost immediately after ice-melt (typically March–April). Even the younger age cohorts (1-year-old), with individual lengths of 110–130 mm LT (body mass about 30–40 g) at stocking in early spring and achieving 160–170 mm LT (about 70–90 g) at the end of June, had by far outgrown the size range available to Little Bitterns. Moreover, large Carp can suppress aquatic insects and amphibians via both trophic and non-trophic effects (Kloskowski 2011). Screens at the water inlets prevented the intrusion of nuisance fish in both types of monoculture ponds, but the large-fish ponds in particular, which had been filled earlier in the season, contained small numbers of small-sized wild fish. (3) Abandoned ponds that belonged to local fish farms, but were left unstocked and hosted diverse and abundant populations of fish available to Little Bitterns, mainly Prussian Carp Carassius gibelio and Topmouth Gudgeon Pseudorasbora parva. Older individuals of large-sized species (including Common Carp) were occasionally observed as well; however, the abundance of large fish appeared to be limited by infrequent draining and/or by winterkill, and the ponds supported significant populations of macroinvertebrates and amphibians (Table 1). (4) Recreational angling ponds stocked with a variety of large-sized fish (mainly Common Carp, Tench Tinca tinca but also piscivorous Pike Esox lucius and Wels Silurus glanis); young, small-sized fish of these species were also stocked on occasion. The large-sized fish were invulnerable to Little Bitterns and could, moreover, adversely impact the non-fish prey of birds. However, rich supplies of small non-cultured fish (mainly Sunbleak Leucaspius delineatus and Topmouth Gudgeon) were present in the ponds (Table 1). As they were considered a food resource for piscivorous sport fish, they were not stopped from entering the ponds. Moreover, the angling ponds were not drained for winter, which facilitated their colonisation and the reproduction of wild-grown fish.
Stable water levels during the breeding season generally prevented nests from being flooded or from being destroyed by terrestrial predators during periods of drought. We cannot rule out local differences in predation risk between the pond clusters; however, owing to their spatial aggregation (Fig. 1), the ponds of all four types, interspersed within the clusters, were subject to similar predation pressure. Hence, we assumed that the four types of ponds differed in food resources for birds with respect to fish management but did not very much in other ecological characteristics that might limit Little Bittern reproduction.
Nest visits and breeding success
The ponds were searched for nests at least twice during each season, in late May–early June and in early July. In late July–early August, a third census was carried out on the ponds with the highest nest densities. We were unlikely to find every single nest on all the ponds; moreover, the whole area of emergent vegetation on a few of the larger ponds was not completely surveyed during each search. Nests were located by wading through the emergent vegetation, marked using GPS, and subsequently revisited at 5-day intervals during incubation and at 2–3-day intervals after egg hatching. Hatching dates were determined on the basis of direct observations of freshly-hatched chicks or back-calculated using growth rates of known-age chicks from the studied population (cf. Pardo-Cervera et al. 2010). The number of chicks reaching the 7th day of life was used as a measure of breeding success, because older chicks can leave the nest and hide in the surrounding vegetation (Cramp and Simmons 1977; Pardo-Cervera et al. 2010; but see Trnka 2020). As individual marking of chicks improved our assessment of their survival to independence, they were fitted with metal and alphanumeric colour rings from about the 7th day of life onwards. To facilitate individual identification (for assessing breeding success) before the nestlings attained a body size appropriate for ringing, their toes were given a unique colour combination with a non-toxic permanent marker. On a less regular basis, adult birds were also ringed with metal and colour rings (enabling possible second broods to be detected), but they were not trapped at the nests to avoid the risk of nest desertion.
Assessment of diet
Young Little Bitterns often regurgitated food when they were measured or ringed. The analysis of regurgitates is the prevailing method for studying the diet of herons (Fasola et al. 1993). However, this method suffers from the biases typical for conventional diet analyses owing to the (uneven) digestion of prey items and its ‘snapshot’ character; nonetheless, compared to other traditional diet sampling techniques, it is non-invasive and digestion is often not advanced (reviewed in Karnovsky et al. 2012). Undigested vertebrates readily identifiable in the field were measured (to 1 mm) and left at the nest or, wherever possible, fed back to the chick to minimise the disturbance in feeding. In the case of fish, the LT measurement was taken, and amphibians were measured from snout to cloaca (longitudo corporis, LC). Remains of strongly digested vertebrates and all macroinvertebrates were frozen for later examination. A total of 326 regurgitates from 78 nests were analysed.
After thawing, the food items were identified under a stereoscopic microscope. If direct identification and measurements were not feasible, prey identity and size were determined using species-specific ‘diagnostic’ bones following the published literature: for fish—Horoszewicz (1960), Libois et al. (1987), Kloskowski et al. (2000) and Beyer et al. (2006); for amphibians—Böhme (1977); and for mammals—Pucek (1984). Taxon-specific keys, e.g. Nilsson (1996, 1997), were used to identify macroinvertebrate prey. Finally, individual prey mass was estimated on the basis of body length–body mass regressions (vertebrates) and size classes (macroinvertebrates) (for details, see the Supplementary Information File: Table S1).
Statistical analyses
The numerical and biomass proportions of prey taxa in the diet of Little Bittern were compared between the four pond habitat types using the non-parametric analysis of similarities (ANOSIM). We pooled all the regurgitates collected from a particular nest and used this as the statistical unit (N = 73 nests). ANOSIM provides a ranking of similarities between observations and ranges from –1 to + 1; positive values indicate bigger differences between groups than within groups. The p value was assessed using 9999 permutations with the random, multiple classification of all observations to groups. ANOSIM allows global analysis for all groups as well as direct comparison in each pair of groups. The Bray–Curtis (dis)similarity index was used in all the analyses (Clarke and Warwick 2001). The analyses were run on two levels of prey taxa classification: (1) three main prey categories (fish, amphibians and macroinvertebrates), (2) with fish and amphibians analysed at species level, and with macroinvertebrates considered as a single group owing to the low proportion of their biomass. The Bonferroni correction for the p value was not applied (Moran 2003). Mammals represented by a single individual were omitted. Owing to the limited representativeness of samples with small numbers of regurgitated prey items, we excluded the few nests from which only 1–2 prey items were retrieved; however, to not underestimate large prey items that individually may have been whole regurgitates, we included nests with such small samples when the total estimated prey biomass exceeded 10 g.
Prey diversity (Shannon–Wiener H) was calculated using the same dataset as for ANOSIM. Vertebrates were analysed at species level and macroinvertebrates at order level. H′ values, the exponential of the Shannon–Wiener index (Jost 2006), were then compared between the four pond types using a generalised linear mixed model (GLMM) with identity link and normal error. Brood hatching date (the date of the first hatched nestling) was entered as a continuous variable to control for potential seasonal changes in prey abundance. Since regurgitates were collected opportunistically during multiple visits, the number of visits during which they were obtained was deemed a random factor to account for uneven sampling.
Whether the importance of fish in the diet increased with brood age was checked using a binomial GLMM with a logit link on the proportion of fish in the food of nestlings (N = 172; the statistical unit used was the total of all regurgitates collected from a nest on the same visit). The number of fish prey items was treated as a binomial response, and the total number of all prey items in the sample was the binomial denominator. The numerical proportion of fish in the regurgitates was a good proxy for biomass proportion (Pearson r = 0.821; p < 0.001). Brood age (the oldest chick’s age on the day the dietary remains were collected) and time of the season (the date of the regurgitate collection) were included as independent continuous variables. To evaluate whether parents caught increasingly larger fish as the chicks grew in size, the body length of the consumed fish was related to brood age using a Gaussian GLMM. The analysis was restricted to fish because of their importance in the chicks’ diet. Since the residuals of fish LT were not normally distributed, log-transformed data were used. To control for the effect of time of season, the brood hatching date was included as a continuous variable; the date of the regurgitate collection was not used because it was correlated with brood age (r = 0.206; p < 0.001). Pond management type was included as a categorical factor in the GLMMs on the proportion and individual size of fish in the diet, and brood identity was used as a random factor, since regurgitates were collected during multiple nest visits.
The Little Bitterns' breeding success between the pond management types was compared using a Gaussian GLMM. Excluded were nests predated (also partially), abandoned or destroyed due to adverse weather conditions or human activities, and broods known to be second ones based on observations of colour-ringed birds (Filipiuk and Kucharczyk 2016). Nesting pond identity and brood hatching date were entered as random factors.
Julian dates were used in the GLMMs, with 1 May set as day 1 (the onset of the breeding season). The nesting phenology was similar in all the study years (range of mean first-clutch laying dates: 28 May–2 June). The significance level was set to α = 0.05. Pearson’s r was applied to check for intercorrelations. As the number of predictors was small, inferences were based on full models (with all fixed terms); minimal significant models produced the same conclusions. Non-significant interactions were dropped. Data normality was tested using the Kolmogorov–Smirnov test. Unless stated otherwise, the results are presented as mean ± standard deviation. Computations were performed using PAST 3.14 (Hammer et al. 2001) and GenStat 15 (VSN International).
Results
General dietary composition
Overall, the Little Bittern’s diet consisted almost exclusively of fish, amphibians and macroinvertebrates (a total of 1356 identified prey items). Fish were dominant in terms of numbers and biomass (66.7% and 83.8%, respectively). Amphibians constituted 9.5% of the prey in terms of numbers and 11.9% in terms of biomass. Mammals were represented by a single Striped Field Mouse Apodemus agrarius. Although macroinvertebrates were taken in considerable numbers (23.7%), their biomass was low (only 3.9% of the total prey). Detailed data on the taxonomic prey composition are given in Table S1.
The chicks’ diet generally consisted of small prey. The mean individual LT of the consumed fish was 45.9 ± 20.7 mm, the body mass was 2.5 g ± 3.9 g (N = 905), and the largest fish recorded was Prussian Carp (118 mm, 31.3 g). The largest amphibian recorded was an adult Pelophylax frog (LC 58 mm, 20 g), but the majority of amphibians consumed were tadpoles and metamorphs. Macroinvertebrate prey items were represented mainly by relatively large-sized insects: Coleoptera, Odonata and Hemiptera: Heteroptera (Table S1).
Feeding ecology and breeding success relative to habitat management
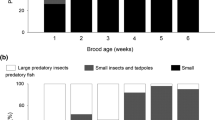
The overall dietary composition differed in terms of numbers of the three main prey categories, i.e. fish, amphibians and macroinvertebrates (ANOSIM: R = 0.107, p = 0.003), but not in terms of biomass (ANOSIM: R = −0.001, p = 0.465; Supplementary Information File: Table S2) between the differently managed ponds. The taxonomically more detailed analysis, with fish and amphibians considered at species level, revealed more obvious differences in numbers (ANOSIM: R = 0.262, p = 0.0001) and biomass (ANOSIM: R = 0.234, p = 0.0001; Table S3; see also Fig. 2).
Common Carp made up more than half of the food biomass (on average 53%) on the monoculture small-fish ponds; amphibians also made a significant contribution (Fig. 2). Notably, Common Carp also featured in the diet of nestlings from the monoculture large-fish ponds (37%), in which cultured Carp were of a size invulnerable to predators. The bulk of the diet (46%) on those ponds was composed of small wild-grown fish (mainly Perch Perca fluviatilis), whilst Prussian Carp and Topmouth Gudgeon collectively constituted the principal prey on the abandoned and angling ponds (57% and 53%, respectively; Fig. 2). As the ponds occurred in clusters, in which differently managed ponds could be situated in close proximity to each other, parent birds were occasionally observed to visit adjacent ponds as well as other small water bodies (ditches and disused water settling tanks) in the vicinity, up to a few hundred metres from the nest.
The H index of food diversity ranged between 0 and 1.96 (mean 1.18 ± 0.46). The GLMM showed no significant influence of pond type or of the time of breeding season on the H′ value (Table S4).
The binomial GLMM showed that the total contribution of all fish species to the chicks’ diet was independent of chick age and of the time of the breeding season, but that it was affected by the type of pond management (Table 2). The proportion of fish in the food was significantly lower on the monoculture small-fish ponds than on the other pond types (Fig. 3).
Back-transformed mean numerical proportions of fish in the Little Bittern diet as predicted by a binomial GLMM in differently managed pond habitats. Pairwise comparisons were based on standard errors of differences of means (SED). The average SED is shown (SED was not back-transformed). The asterisk denotes significant differences between monoculture small-fish ponds and the other pond types (p < 0.05). Sample sizes are given in the columns. For more details, see Table 2
Fish prey size was strongly related to chick age—the older the chicks, the larger the fish they were fed (Table 3), although remains of a few relatively large fish (80–100 mm LT) were found in regurgitates collected from young (1–3 days old) chicks. The analysis showed no significant influence of pond type or of the time of the breeding season on the size of the prey taken (Table 3).
The overall mean reproductive output of successful nests (i.e. with at least one chick reaching the 7th day of life) in our study area was 4.71 ± 0.99 chick per brood. No significant influence of nesting pond type on breeding success was found (Table 4).
Discussion
Prey composition and its determinants
In general, fish were dominant in the food delivered to young Little Bitterns by their parents on the studied ponds, this is consistent with the results of other studies (Holmes and Hatchwell 1991; Melikyan 2008; Pardo-Cervera et al. 2010; Trnka 2020). According to optimal foraging theory (Krebs and McCleery 1984), the high contribution of fish to the diet of chicks is energetically advantageous, since the nutritive and energetic values of fish are relatively high (Nelson and Kruuk 1997; Ledwoń and Neubauer 2017) and should, thus, be preferred to other aquatic prey. The contribution of (mainly pre-metamorphosed) amphibians, energetically less profitable than fish (Nelson and Kruuk 1997), was clearly lower (but see Flis and Gwiazda 2018). However, given the rapid digestion rate in herons (Vinokurov 1960), regurgitate analysis is likely to underestimate soft-bodied prey (Fasola et al. 1993; Karnovsky et al. 2012) such as amphibian larvae. The small contribution of adult amphibians was presumably due to their body size often exceeding the swallowing abilities of nestlings, even though anurans may feature heavily in the diet of adult birds (see Kayser 2010). Despite their high numerical proportion, the contribution of macroinvertebrates in terms of biomass was minimal because of their relatively small body size. The core of the macroinvertebrate prey consisted of the larvae of dytiscid water beetles and anisopteran dragonflies, the largest aquatic insects in the study area; however, some of the insects recorded in the regurgitates (e.g. adult Hydrophilidae) were very small, so one cannot rule out that they were secondary prey items originally ingested by predatory fish or amphibians subsequently taken by Little Bitterns.
The results reveal the strong influence of pond management on the taxonomic composition of the Little Bitterns’ diet. However, the proportions of the main prey categories in terms of biomass were similar. This may indicate that Little Bitterns forage opportunistically, but even when alternative prey is abundant, the high proportion of the most profitable food (in this case, fish) remains fairly stable. The dietary contribution of cultured Common Carp was the highest on the monoculture small-fish ponds, obviously due to its size availability. On the other hand, the total proportion of fish in the food was the lowest there (although still clearly exceeding other prey in the regurgitates), showing that non-fish prey was readily taken when highly abundant in the ponds. Non-fish prey made a markedly smaller contribution to the diet of nestlings on the monoculture large-fish ponds. Since the cultured fish were too big for Little Bitterns, small wild fish prevailed in their diet. The considerable dietary contribution of small, young-of-the-year Common Carp (entirely absent from the large-fish ponds) points to a certain plasticity in foraging; clearly, parents flew to other ponds for food, presumably because of the poor food resources of the large-fish ponds. These ponds, seemingly attractive to Little Bitterns because of the otherwise appropriate nesting habitat (extensive areas of emergent vegetation and water levels suitable for breeding), may act as an ecological trap resulting from shortages, unforeseen by the parent birds, of prey items small enough to be fed to chicks (cf. Kloskowski 2012). Although we did not quantify the effort involved in searching for nests in each pond type, we found relatively very few nests on the monoculture ponds with large fish, which indicates that breeding Little Bitterns may generally avoid nesting on ponds strongly dominated by large fish.
As in the monoculture large-fish ponds, large fish were to a great extent able to reduce the abundance of prey they shared with birds in the angling ponds (cf. Wood et al. 2001). However, the pond operators encouraged the presence of small wild species or young sport fish; obviously, these were also available to the Little Bitterns. The prey biomass composition of nestlings on the abandoned ponds was similar to that on the angling ponds, the principal prey being Prussian Carp and Topmouth Gudgeon (see also Trnka 2020), although the abundance of amphibians and macroinvertebrates in the abandoned ponds was evidently higher. Prussian Carp and Topmouth Gudgeon were not intentionally stocked as both species are invasive in Europe (van der Veer and Nentwig 2015).
Pond management did not influence dietary diversity: this was rather unexpected, considering the effects of different stocking practices on prey abundance. Although we could not compare prey diversity amongst the pond types, it is likely that at lower taxonomic levels, it was generally similar. For example, in ponds where macroinvertebrate and amphibian densities were greatly diminished by large fish, the overall diversity was maintained by the species richness of small fish. The dietary diversity could also have been increased by parent birds making foraging trips to other ponds.
The dietary composition and the availability of fish prey lead to the conclusion that in the system studied here, consisting of differently managed ponds, Little Bitterns found favourable provisioning conditions on the abandoned and the extensively cultured ponds with high abundances of small fish. Pond management targeting anglers appears to be able to provide sufficient food resources, too, despite competition from large sport fish (see also Trnka 2020). However, this study assessed the dietary composition, not the total biomass or amount of energy delivered to nestlings. The high proportion of fish in the diet does not rule out poor food conditions in which fish, though being the most accessible prey, are actually scarce. Consequently, the considerable dietary proportion of highly profitable prey does not preclude chicks from being undernourished (see: Lõhmus and Väli 2004; Kloskowski et al. 2021). Overall, fish ponds appear to offer attractive breeding habitats for Little Bitterns. Despite the widespread use of managed habitats (Keller et al. 2020), this small heron is unlikely to come into conflict with human economic interests, because its gape limitation restricts any impact to the earliest stages of fish production. In outdoor pond cultures, the natural mortality of young fish is usually high (Lorenzen 1996) and does not imply serious financial losses. Moreover, the exploitation of undesirable wild fish invading aquaculture ponds, such as Prussian Carp and Topmouth Gudgeon in our system, may go some way to compensate for potential damage to cultured fish (cf. Ashkenazi and Yom-Tow 1996).
Breeding success
The predicted differences in breeding success between ponds differing in management practices were not observed. The results are likely to have been biased by parental foraging beyond the nesting pond, which may have enabled the Little Bitterns to compensate for the scarcity of prey in the ponds dominated by large fish. Foraging flights beyond the nesting area can be an important component of the provisioning strategy of parent Little Bitterns in sub-optimal habitats (see Pezzo and Benocci 2001), but they are energetically disadvantageous and increase the risk of predation on the foraging parents as well as on the less closely attended broods (Eberl and Picman 1993). The costs of nesting in food-poor habitats are relatively easily reduced when habitats differing in prey availability are spatially aggregated, i.e. when foraging trips to other habitat patches do not require long flights, as was the case in this study, where ponds of different types were interspersed and close to each other. Overall, the production of Little Bittern chicks on cyprinid fish ponds seems to be high (see also Cempulik 1994; Trnka 2020), but comparisons with other habitats are difficult owing to the different measures of reproductive success applied (e.g. Martínez-Abraín 1994; Pardo-Cervera et al. 2010; but see Holmes and Hatchwell 1991). Data on the Little Bittern’s breeding success are usually limited to the 1st week post-hatching, whereas the chicks' energy demands continue to grow in the period when they are able to leave the nest. Thus, the competitive effect of large fish may be more pronounced in the later stages of chick growth, affecting the near-fledging young which are starting to forage on their own whilst they are still confined to the natal pond. The inexperienced young may be particularly vulnerable to the scarcity of small prey items: compared to adults, juvenile herons are known to exploit smaller prey (Papakostas et al. 2005).
Age-dependent provisioning strategy
As the chicks grow, the parents have to meet the increasing energy demands of their offspring. According to the central-place foraging theory, provisioning large prey (relative to the chicks’ ingesting ability) reduces the time and effort needed for foraging and also the frequency of nest visits, and thus maximises the predators’ net energy gain (Orians and Pearson 1979). Selective provisioning of small prey to hatchlings at the early brood stage can be challenging (Reimchen and Douglas 1985); however, the energetic costs of the effort required to capture sufficient amounts of small prey are mitigated in herons by their ability to deliver multiple-prey loads (Orians and Pearson 1979). Alternatively, the pre-digestion of large-sized prey rather than its size adjustment can help to overcome constraints posed on parent herons by the gape limitation of their nestlings (Owen and Phillips 1956; Kushlan 1978; Voisin 1991). Feeding partly digested food to chicks is advantageous, especially when small-sized prey is scarce, but the process of digestion can be energy- and time-consuming, which may reduce the feeding frequency (Moser 1986). The presence of some exceptionally large-sized prey items in the hatchlings’ diet indicates that pre-digestion facilitated parental provisioning, but the Little Bitterns obviously adjusted the prey size to the ingesting ability of their chicks by progressively increasing the size of the prey delivered. The proportion of fish in the diet did not change either with chick growth or with the progress of the season, although seasonal changes in food composition of nestlings have been documented in other herons (Fasola et al. 1993; Delord et al. 2004).
In conclusion, differing nestling diet compositions in response to management-induced variation in pond prey communities are indicative of foraging plasticity in the Little Bittern (see also Trnka 2020). Nevertheless, in fish-dominated habitats, chick provisioning depends mostly on fish, and small-sized fish may be a critical prey resource during the early brood stage as parents adjust the prey size to the growth-related ingesting capabilities of their young. Some of the habitats offered by cyprinid ponds do not meet the breeding requirements of piscivorous birds because of the shortage of size-available prey. However, cyprinid farms are typically heterogeneous clusters of ponds stocked with different-age cohorts (Horváth et al. 1992), and birds nesting on food-poor ponds have good access to the diverse food resources of adjacent ponds. Given the widespread anthropogenic degradation of natural wetlands, traditional cyprinid culture in ponds can offer important substitute habitats where Little Bitterns are capable of attaining a high level of reproductive success. Similarly, management targeting Little Bittern protection in habitats in which fish are not commercially cultured and harvested should, apart from maintaining a stable water level and vegetation cover (e.g. Voisin 1991; Pardo-Cervera 2010), ensure abundant prey small enough for consumption at the brood stage. It may also be necessary to counteract the establishment of fish species capable of attaining large sizes. We recommend a network approach to the conservation of complex wetland areas, in which different-quality habitat patches, including both managed and unmanaged waters, irrespective of their individual quality, are aggregated and affect the attractiveness of adjacent patches (Resetarits et al. 2005). Given the Little Bittern’s foraging mobility (Pezzo and Benocci 2001), patches suitable for nesting yet with poor food resources may be still attractive and successfully occupied, provided that prey-rich sites are situated close by.
Availability of data
Available from the first author on reasonable request.
References
Ashkenazi S, Yom-Tov Y (1996) Herons and fish farming in the Huleh Valley, Israel: conflict or mutual benefit? Colon Waterbirds 19:143–151
Beyer K, Miranda R, Copp GH, Gozlan RE (2006) Biometric data and bone identification of topmouth gudgeon, Pseudorasbora parva and sunbleak, Leucaspius delineatus. Folia Zool 55:287–292
Böhme G (1977) Zur Bestimmung quartärer Anuren Europas an Hand von Skelettelementen. Wiss Z Humb-Uni Berlin, Math-Nat R 26:283–300
Bryan AL, Coulter MC, Pennycuick CJ (1995) Foraging strategies and energetic costs of foraging flights by breeding wood storks. Condor 97:133–140
Campos F, Lekuona JM (1997) Temporal variations in the feeding habits of the Purple Heron Ardea purpurea during the breeding season. Ibis 139:447–451
Cempulik P (1994) Bestandsentwicklung, Brutbiologie und Ökologie der Zwergdommel Ixobrychus minutus an Fisch- und Industrieteichen Oberschlesiens. Vogelwelt 115:19–27
Cheng C, Liu J, Ma Z (2022) Effects of aquaculture on the maintenance of waterbird populations. Conserv Biol 36:e13913
Clarke KR, Warwick RM (2001) Changes in marine communities: an approach to statistical analysis and interpretation. PRIMER-E Plymouth Marine Laboratory, Plymouth
Cramp S, Simmons KEL (1977) Handbook of the Birds of Europe, the Middle East and North Africa. The Birds of the Western Palearctic. Vol. 1: Ostrich to Ducks. Oxford University Press, Oxford
Delord K, Kayser Y, Cohez D, Befeld S, Hafner H (2004) Fluctuations in chick diet of the Squacco Heron Ardeola ralloides in southern France: changes over the last 30 years. Bird Study 51:69–75
Dudgeon D, Arthington AH, Gessner MO, Kawabata Z-I, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard A-H, Soto D, Stiassny MLJ, Sullivan CA (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182
Eberl C, Picman J (1993) Effect of nest-site location on reproductive success of Red-throated Loons (Gavia stellata). Auk 110:436–444
Eriksson MOG (1986) Reproduction of Black-throated Diver Gavia arctica in relation to fish density in oligotrophic lakes in southwestern Sweden. Ornis Scand 17:245–248
Fasola M, Rosa P, Canova I (1993) The diets of Squacco Herons, Little Egrets, Night, Purple and Grey Herons in their Italian breeding ranges. Rev Ecol - Terre Vie 48:35–47
Fernández Ajó AA, Gatto A, Yorio P (2011) Patterns of prey provisioning in relation to chick age in the South American tern (Sterna hirundinacea). Ornitol Neotrop 22:361–368
Filipiuk M, Kucharczyk M (2016) A puzzling case of successful double-brooding in the Little Bittern Ixobrychus m. minutus. Ardea 104:182–186
Flis A, Gwiazda R (2018) Diet and feeding of nestling Little Bitterns Ixobrychus minutus at fishponds: testing a new method for studying a difficult-to-monitor species. Bird Study 65:257–260
Haas K, Köhler U, Diehl S, Köhler P, Dietrich S, Holler S, Jaensch A, Niedermaier M, Vilsmeier J (2007) Influence of fish on habitat choice of water birds: a whole system experiment. Ecology 88:2915–2925
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeon Elect 4:9
Hampl R, Bureš S, Baláž P, Bobek M, Pojer F (2005) Food provisioning and nestling diet of the black stork in the Czech Republic. Waterbirds 28:35–40
Holmes PR, Hatchwell BJ (1991) Notes on the ecology of the Little Bittern Ixobrychus minutus at Haigam Rakh, Kashmir, India. Forktail 6:25–33
Horoszewicz L (1960) Wartości kości gardłowych dolnych (ossa pharyngea interiora) jako kryterium gatunkowego oznaczania ryb karpiowatych (Cyprynidae). Rocz Nauk Roln B 75:237–258
Horváth L, Tamás G, Seagrave C (1992) Carp and pond fish culture. Oxford University Press, Oxford
Hyvönen T, Nummi P (2000) Activity traps and the corer: complementary methods for sampling aquatic invertebrates. Hydrobiologia 432:121–125
Jackson DB (2003) Between-lake differences in the diet and provisioning behaviour of black-throated divers Gavia arctica breeding in Scotland. Ibis 145:30–44
Jost L (2006) Entropy and diversity. Oikos 113:363–375
Karnovsky NJ, Hobson KA, Iverson SJ (2012) From lavage to lipids: estimating diets of seabirds. Mar Ecol Prog Ser 451:263–284
Kayser Y (2010) Quelques données sur l’alimentation du Blongios nain Ixobrychus minutus en Camargue, sud de la France. Nos Oiseaux 57:277–280
Keller V, Herrando S, Voříšek P, Franch M, Kipson M, Milanesi P, Martí D, Anton M, Klvaňová A, Kalyakin MV, Bauer H-G, Foppen RPB (2020) European Breeding Bird Atlas 2: Distribution, Abundance and Change. European Bird Census Council & Lynx Edicions, Barcelona
Kim M, Yoo J (2012) Diet of yellow bitterns (Ixobrychus sinensis) during the breeding season in South Korea. J Ecol Field Biol 35:9–14
Kloskowski J (2004) Food provisioning in red-necked grebes (Podiceps grisegena) at common carp (Cyprinus carpio) ponds. Hydrobiologia 525:131–138
Kloskowski J (2011) Consequences of the size structure of fish populations for their effects on a generalist avian predator. Oecologia 166:517–530
Kloskowski J (2012) Fish stocking creates an ecological trap for an avian predator via effects on prey availability. Oikos 121:1567–1576
Kloskowski J, Grendel A, Wronka M (2000) The use of fish bones of three farm fish species in diet analysis of the Eurasian otter, Lutra lutra. Folia Zool 49:183–190
Kloskowski J, Nieoczym M, Polak M, Pitucha P (2010) Habitat selection by breeding waterbirds at ponds with size-structured fish populations. Naturwissenschaften 97:673–682
Kloskowski J, Trembaczowski A, Filipiuk M (2021) Higher reproductive performance of a piscivorous avian predator feeding on lower trophic-level diets on ponds with shorter food chains. J Ornithol 160:1049–1062
Krebs JR, McCleery RH (1984) Optimization in behavioural ecology. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach. Blackwell Scientific Publications, Oxford, pp 91–121
Kushlan JA (1978) Feeding ecology of wading birds. In: Sprunt A, Ogden JC, Winckler S (eds) Wading birds. National Audubon Society, New York, pp 249–297
Ledwoń M, Neubauer G (2017) Offspring desertion and parental care in the Whiskered Tern Chlidonias hybrida. Ibis 159:860–872
Libois RM, Hallet-Libois C, Rosoux R (1987) Éléments pour l’indentification des restes craniens des poissons dulçaquicoles de Belgique et du nord de la France: 1—Anguilliformes, Gastérostéiformes, Cyprinodontiformes et Perciformes. Fiches D’ostéologie Animale Pour L’archéologie A 3:1–15
Lõhmus A, Väli Ü (2004) The effects of habitat quality and female size on the productivity of the lesser spotted eagle Aquila pomarina in the light of the alternative prey hypothesis. J Avian Biol 35:455–464
Lorenzen K (1996) The relationship between body weight and natural mortality in juvenile and adult fish: a comparison of natural ecosystems and aquaculture. J Fish Biol 49:627–642
Ma Z, Cai Y, Li B, Chen J (2010) Managing wetland habitats for waterbirds: an international perspective. Wetlands 30:15–27
Maceda-Veiga A, López R, Green AJ (2017) Dramatic impact of alien carp Cyprinus carpio on globally threatened diving ducks and other waterbirds in Mediterranean shallow lakes. Biol Conserv 212:74–85
Martin TE (1987) Food as a limit on breeding birds: A life-history perspective. Annu Rev Ecol Syst 18:453–487
Martínez-Abraín A (1994) Nota sobre la biologia de Ixobrychus m. minutus (L.) durante el periodo de reproduccion en Valencia (E, España). Ardeola 41:169–171
Melikyan KA (2008) Biologiya gnezdovaniya maloy vypi Ixobrychus minutus (L.) na rybovodnykh prudakh Araratskoy Ravniny. Biol J Armen 60:34–44
Moltoni E (1948) L’alimentazione degli Ardeidae (Aironi) in Italia. Riv Ital Ornitol 18:87–93
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100:403–405
Moser ME (1986) Prey profitability for adult Grey Herons Ardea cinerea and the constraints on prey size when feeding young nestlings. Ibis 128:392–405
Nelson K, Kruuk H (1997) The prey of otters: calorific content of eels (Anguilla anguilla) and other fish, frogs (Rana temporaria) and toads (Bufo bufo). IUCN Otter Spec Group Bull 14:75–80
Nieoczym M, Kloskowski J (2015) Responses of epibenthic and nektonic macroinvertebrate communities to a gradient of fish size in ponds. J Limnol 74:50–62
Nilsson A (ed) (1996) Aquatic Insects of North Europe. A taxonomic handbook. Vol. 1. Ephemeroptera—Plecoptera - Heteroptera—Megaloptera—Neuroptera—Coleoptera—Trichoptera—Lepidoptera. Apollo Books, Stenstrup
Nilsson A (ed) (1997) Aquatic Insects of North Europe. A taxonomic handbook. Vol. 2. Odonata—Diptera. Apollo Books, Stenstrup
Nummi P, Väänänen VM, Holopainen S, Pöysä H (2016) Duck-fish competition in boreal lakes—a review. Ornis Fennica 93:67–76
Orians GH, Pearson NE (1979) On the theory of central place foraging. In: Horn DJ, Stairs BR, Mitchell RD (eds) Analysis of ecological systems. Ohio State University Press, Columbus, pp 155–177
Owen DF, Phillips GC (1956) The food of nestling Purple Herons in Holland. Brit Birds 49:494–499
Papakostas G, Kazantzidis S, Goutner V, Charalambidou I (2005) Factors affecting the foraging behavior of Squacco Heron. Waterbirds 28:28–34
Pardo-Cervera F, Sørensen IH, Jensen C, Ruiz X, Sanchez-Alonso C (2010) Breeding biology of the Little Bittern Ixobrychus minutus in the Ebro delta (NE Spain). Ardeola 57:407–416
Pezzo F, Benocci A (2001) Spatial behaviour of the Little Bittern Ixobrychus minutus, implications for conservation. Avocetta 25:78
Pucek Z (1984) Klucz do oznaczania ssaków Polski. PWN, Warszawa
Reimchen TE, Douglas S (1985) Differential Contribution of the Sexes to Prefledged Young in Red-Throated Loons. Auk 102:198–201
Resetarits WJ Jr, Binckley CA, Chalcraft DR (2005) Habitat selection, species interactions, and processes of community assembly in complex landscapes. In: Holyoak M, Leibold MA, Holt R (eds) Metacommunities: spatial dynamics and ecological communities. University of Chicago Press, Chicago, pp 374–398
Stienen EWM, van Beers PWM, Brenninkmeijer A, Habraken JMPM, Raaijmakers MHJE, van Tienen PGM (2000) Reflections of a specialist: patterns in food provisioning and foraging conditions in Sandwich Terns Sterna sandvicensis. Ardea 88:33–49
Švažas S, Raudonikis L, Stanevičius V (2000) Importance of large fish-pond systems for rare breeding bird species and migratory water-bird populations in Lithuania. Sylvia 36(Supplement):21
Tomiałojć L, Stawarczyk T (2003) Awifauna Polski. Rozmieszczenie, liczebność i zmiany. PTPP „pro Natura”, Wrocław, pp 84–87
Trnka A (2020) Nestling diet and breeding success of Little Bitterns Ixobrychus minutus at two artificial fishpond complexes in south-western Slovakia. Ardea 108:161–169
van der Veer G, Nentwig W (2015) Environmental and economic impact assessment of alien and invasive fish species in Europe using the generic impact scoring system. Ecol Freshw Fish 24:646–656
Vasvari N (1929) Beiträge zur Ernährungsökologie von Botaurus stellaris L. und Ardetta minuta L. Aquila 34–35:342–374
Vinokurov AA (1960) On the food digestion rate in herons. Moskovske Obshchestvo Ispryatelei Prirody Bjulletin Otdel Biologii Moskva 65:10
Voisin C (1991) The herons of Europe. Poyser, London
Wood PJ, Greenwood MT, Barker SA, Gunn J (2001) The effects of amenity management for angling on the conservation value of aquatic invertebrate communities in old industrial ponds. Biol Conserv 102:17–29
Acknowledgements
We thank Żaneta Lachowska-Filipiuk, Magdalena Deruś, Adam Flis and other volunteers for their field assistance. The comments of two anonymous reviewers have improved the manuscript. This work was approved by the Regional Directorate for Environmental Protection in Lublin (WPN.6401.117.2014.MPR; WPN.6401.117.2014.MPR.1; WPN.6401.261.2015.MPR).
Funding
The research was partially supported by Grants for Young Scientists awarded to Maciej Filipiuk by the Institute of Biological Sciences of the Maria Curie-Skłodowska University in Lublin (PSP: BS-M-11-010-15-1-19, PSP: BS-M-11-010-16-1-18, PSB: BS-M-11-010-17-1-13).
Author information
Authors and Affiliations
Contributions
Conceptualization: MF, JK; field work: MF, JK; analyses of collected food material: MF, PB; statistical analyses: JK, MF; writing—original draft preparation: MF, JK; writing—review and editing: MF, JK, PB.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Animal welfare statement
No animals were harmed during the study.
Additional information
Communicated by C. Barbraud.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Filipiuk, M., Buczyński, P. & Kloskowski, J. Feeding ecology and reproductive success of the Little Bittern Ixobrychus minutus in differently managed pond habitats. J Ornithol 165, 473–484 (2024). https://doi.org/10.1007/s10336-023-02119-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02119-y