Abstract
Objective
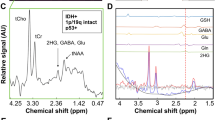
Oncometabolite D-2-hydroxyglutarate (2HG) is pooled in isocitrate dehydrogenase (IDH)-mutant glioma cells. Detecting 2HG by MR spectroscopy (MRS) has been proven viable in the last decade but has not entirely found its way into the clinical routine. This study aimed to explore the adoption of 2HG MRS while acknowledging factors that influence its performance in the clinical environment.
Methods
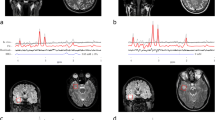
Thirty-nine MR spectra were acquired and reported prospectively in patients with suspected glioma using a 3 T system with Mescher–Garwood point-resolved spectroscopy (MEGA-PRESS) sequence utilizing averaged free induction decay (FID) signals. Postprocessing and evaluation of spectra were performed with jMRUI and LCModel. 2HG concentration estimates, 2HG/Cr ratio, together with quality measures, including Cramér–Rao lower bounds (CRLBs), full-width at half-maximum (FWHM) values, and signal-to-noise ratio (SNR) were calculated using LCModel. Immunohistochemistry and genomic analysis results used as a ground truth were available for 15 patients.
Results
The threshold for test positivity was set according to the ROC curve at 1 mM. Calculated sensitivity was 57.14% (95% CI 0.20–0.88), specificity 87.5% (95% CI 0.46–0.99), positive predictive value 80%, and negative predictive value 70%. Overall diagnostic accuracy was 73.33% (95% CI 0.45–0.92). The 2HG/Cr ratio with the cutoff value 0.085 significantly improved sensitivity and overall diagnostic accuracy [85.71%, 95% CI 0.42–1.00 and 86.67%, (95% CI 0.60–0.98), respectively].
Conclusion
Multiple factors compromising spectral quality in the clinical adoption of edited 2HG MRS resulted in diminished sensitivity but clinically acceptable specificity. Furthermore, the 2HG/Cr ratio performs better than the sole 2HG concentration estimate in the pre-operative setting.


Similar content being viewed by others
References
Reitman ZJ, Jin G, Karoly ED et al (2011) Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci 108:3270–3275. https://doi.org/10.1073/pnas.1019393108
Dang L, Yen K, Attar EC (2016) IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol 27:599–608. https://doi.org/10.1093/annonc/mdw013
Liu X, Gong Y (2019) Isocitrate dehydrogenase inhibitors in acute myeloid leukemia. Biomark Res 7:22. https://doi.org/10.1186/s40364-019-0173-z
Amary MF, Bacsi K, Maggiani F et al (2011) IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathology 224:334–343. https://doi.org/10.1002/path.2913
Salati M, Caputo F, Baldessari C et al (2020) IDH signalling pathway in cholangiocarcinoma: from biological rationale to therapeutic targeting. Cancers 12:3310. https://doi.org/10.3390/cancers12113310
Pansuriya TC, van Eijk R, d’Adamo P et al (2011) Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet 43:1256–1261. https://doi.org/10.1038/ng.1004
Network TCGAR, Abeshouse A, Ahn J et al (2015) The molecular taxonomy of primary prostate cancer. Cell 163:1011–1025. https://doi.org/10.1016/j.cell.2015.10.025
Raineri S, Mellor J (2018) IDH1: linking metabolism and epigenetics. Front Genet 9:493. https://doi.org/10.3389/fgene.2018.00493
Sulkowski PL, Corso CD, Robinson ND et al (2017) 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med 9:eaal2463. https://doi.org/10.1126/scitranslmed.aal2463
Tejera D, Kushnirsky M, Gultekin SH et al (2020) Ivosidenib, an IDH1 inhibitor, in a patient with recurrent, IDH1-mutant glioblastoma: a case report from a phase I study. CNS Oncol 9:CNS62. https://doi.org/10.2217/cns-2020-0014
Wei S, Zhao J (2017) 2016 World health organization classification of tumors of the central nervous system: an interpretation. Chin J Neuromed 16:529–536. https://doi.org/10.3760/cma.j.issn.1671-8925.2017.05.018
Youssef G, Miller JJ (2020) Lower grade gliomas. Curr Neurol Neurosci 20:21. https://doi.org/10.1007/s11910-020-01040-8
Suh CH, Kim HS, Jung SC et al (2018) 2-Hydroxyglutarate MR spectroscopy for prediction of isocitrate dehydrogenase mutant glioma: a systemic review and meta-analysis using individual patient data. Neuro Oncol 20:1573–1583. https://doi.org/10.1093/neuonc/noy113
Branzoli F, Stefano ALD, Capelle L et al (2017) Highly specific determination of IDH status using edited in vivo magnetic resonance spectroscopy. Neuro Oncol 20:907–916. https://doi.org/10.1093/neuonc/nox214
Kim H, Kim S, Lee HH, Heo H (2016) In-vivo proton magnetic resonance spectroscopy of 2-hydroxyglutarate in isocitrate dehydrogenase-mutated gliomas: a technical review for neuroradiologists. Korean J Radiol 17:620–632. https://doi.org/10.3348/kjr.2016.17.5.620
Choi C, Ganji SK, DeBerardinis RJ et al (2012) 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med 18:624–629. https://doi.org/10.1038/nm.2682
Starčuk Z, Starčuková J (2017) Quantum-mechanical simulations for in vivo MR spectroscopy: Principles and possibilities demonstrated with the program NMRScopeB. Anal Biochem 529:79–97. https://doi.org/10.1016/j.ab.2016.10.007
Naressi A, Couturier C, Devos JM et al (2001) Java-based graphical user interface for the MRUI quantitation package. Magn Reson Mater Phys Biol Med 12:141–152. https://doi.org/10.1016/s1352-8661(01)00111-9
Stefan D, Cesare FD, Andrasescu A et al (2009) Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas Sci Technol 20:104035. https://doi.org/10.1088/0957-0233/20/10/104035
Govindaraju V, Young K, Maudsley AA (2000) Proton NMR chemical shifts and coupling constants for brain metabolites. Nmr Biomed 13:129–153. https://doi.org/10.1002/1099-1492(200005)13:3%3c129::aid-nbm619%3e3.0.co;2-v
Kaiser LG, Marjańska M, Matson GB et al (2010) 1H MRS detection of glycine residue of reduced glutathione in vivo. J Magn Reson 202:259–266. https://doi.org/10.1016/j.jmr.2009.11.013
Wilson M (2021) spant: an R package for magnetic resonance spectroscopy analysis. J Open Source Softw 6:3646. https://doi.org/10.21105/joss.03646
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679. https://doi.org/10.1002/mrm.1910300604
Choi C, Raisanen JM, Ganji SK et al (2016) Prospective longitudinal analysis of 2-hydroxyglutarate magnetic resonance spectroscopy identifies broad clinical utility for the management of patients with IDH-mutant glioma. J Clin Oncol 34:4030–4039. https://doi.org/10.1200/jco.2016.67.1222
Nagashima H, Tanaka K, Sasayama T et al (2016) Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for IDH1 mutant glioma. Neuro Oncol 18:1559–1568. https://doi.org/10.1093/neuonc/now090
de la Fuente MI, Young RJ, Rubel J et al (2016) Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro Oncol 18:283–290. https://doi.org/10.1093/neuonc/nov307
Costanzo AD, Trojsi F, Popolizio T et al (2006) High field brain MRI. Use Clin Pract. https://doi.org/10.1007/3-540-31776-7_18
Andronesi OC, Kim GS, Gerstner E et al (2012) Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med 4:116ra4. https://doi.org/10.1126/scitranslmed.3002693
Crisi G, Filice S, Michiara M et al (2018) 2-Hydroxyglutarate detection by short echo time magnetic resonance spectroscopy in routine imaging study of brain glioma at 3.0 T. J Comput Assist Tomogr 42:469–474. https://doi.org/10.1097/rct.0000000000000705
Tietze A, Choi C, Mickey B et al (2018) Non-invasive assessment of isocitrate dehydrogenase mutation status in cerebral gliomas by magnetic resonance spectroscopy in a clinical setting. J Neurosurg 128:391–398. https://doi.org/10.3171/2016.10.jns161793
Zhou M, Zhou Y, Liao H et al (2018) Diagnostic accuracy of 2-hydroxyglutarate magnetic resonance spectroscopy in newly diagnosed brain mass and suspected recurrent gliomas. Neuro Oncol 20:1262–1271. https://doi.org/10.1093/neuonc/noy022
Liu A, Hou C, Chen H et al (2016) Genetics and epigenetics of glioblastoma: applications and overall incidence of IDH1 mutation. Front Oncol 6:16. https://doi.org/10.3389/fonc.2016.00016
Liao Y, Oros-Peusquens A-M, Lindemeyer J et al (2019) An MR technique for simultaneous quantitative imaging of water content, conductivity and susceptibility, with application to brain tumours using a 3T hybrid MR-PET scanner. Sci Rep UK 9:88. https://doi.org/10.1038/s41598-018-36435-8
Gu W, Fang S, Hou X et al (2021) Exploring diagnostic performance of T2 mapping in diffuse glioma grading. Quant Imaging Med Surg 11:2942954–2943954. https://doi.org/10.21037/qims-20-916
Kern M, Auer TA, Picht T et al (2020) T2 mapping of molecular subtypes of WHO grade II/III gliomas. Bmc Neurol 20:8. https://doi.org/10.1186/s12883-019-1590-1
Acknowledgements
The MRS package was developed by Edward J. Auerbach and Małgorzata Marjańska and provided by the University of Minnesota under a C2P agreement. The authors would like to thank Małgorzata Marjańska and Marek Chmelík for guidance in data acquisition and helpful discussions.
Funding
No funding was received for conducting this study. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
DJ: study conception and design, analysis and interpretation of data, and drafting of the manuscript. JPM: study conception and design, and drafting of the manuscript. SH: acquisition of data, analysis, and interpretation of data. LP: analysis and interpretation of data, and critical revision. KK: resection of tumors. MS: ex vivo tissue analysis.
Corresponding author
Ethics declarations
Ethical standards
This study was approved by the Ethical Committee of Faculty Hospital in Nitra, which complies with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Juskanič, D., Mištinová, J.P., Hollý, S. et al. Diagnostic performance of edited 2HG MR spectroscopy of central glioma in the clinical environment. Magn Reson Mater Phy 35, 45–52 (2022). https://doi.org/10.1007/s10334-021-00989-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-021-00989-y