Abstract
Objectives
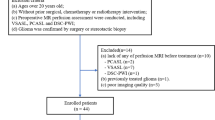
To investigate the repeatability of perfusion measures in gliomas using pulsed- and pseudo-continuous-arterial spin labelling (PASL, PCASL) techniques, and evaluate different regions-of-interest (ROIs) for relative tumour blood flow (rTBF) normalisation.
Materials and methods
Repeatability of cerebral blood flow (CBF) was measured in the Contralateral Normal Appearing Hemisphere (CNAH) and in brain tumours (aTBF). rTBF was normalised using both large/small ROIs from the CNAH. Repeatability was evaluated with intra-class-correlation-coefficient (ICC), Within-Coefficient-of-Variation (WCoV) and Coefficient-of-Repeatability (CR).
Results
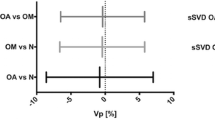
PASL and PCASL demonstrated high reliability (ICC > 0.9) for CNAH-CBF, aTBF and rTBF. PCASL demonstrated a more stable signal-to-noise ratio (SNR) with a lower WCoV of the SNR than that of PASL (10.9–42.5% vs. 12.3–29.2%). PASL and PCASL showed higher WCoV in aTBF and rTBF than in CNAH CBF in WM and GM but not in the caudate, and higher WCoV for rTBF than for aTBF when normalised using a small ROI (PASL 8.1% vs. 4.7%, PCASL 10.9% vs. 7.9%, respectively). The lowest CR was observed for rTBF normalised with a large ROI.
Discussion
PASL and PCASL showed similar repeatability for the assessment of perfusion parameters in patients with primary brain tumours as previous studies based on volunteers. Both methods displayed reasonable WCoV in the tumour area and CNAH. PCASL’s more stable SNR in small areas (caudate) is likely to be due to the longer post-labelling delays.









Similar content being viewed by others
Notes
http://qibawiki.rsna.org/images/8/8c/FMRITechnicalPerformanceIndices041613.pdf, accessed on June 2018.
References
Alsaedi A, Doniselli F, Jäger HR, Panovska-Griffiths J, Rojas-Garcia A, Golay X, Bisdas S (2019) The value of arterial spin labelling in adults glioma grading: systematic review and meta-analysis. Oncotarget (Internet) 10:1589–1601. https://doi.org/10.18632/oncotarget.26674
Kong L, Chen H, Yang Y, Chen L (2017) A meta-analysis of arterial spin labelling perfusion values for the prediction of glioma grade. Clin Radiol (Internet; The Royal College of Radiologists). 72:255–261. https://doi.org/10.1016/j.crad.2016.10.016
Weber MA, Thilmann C, Lichy MP, Günther M, Delorme S, Zuna I, Bongers A, Schad LR, Debus J, Kauczor HU, Essig M, Schlemmer HP (2004) Assessment of irradiated brain metastases by means of arterial spin-labeling and dynamic susceptibility-weighted contrast-enhanced perfusion MRI: initial results. Invest Radiol 39:277–287. https://doi.org/10.1097/01.rli.0000119195.50515.04
Yun TJ, Cho HR, Choi SH, Kim H, Won JK, Park SW, Kim JH, Sohn CH, Han MH (2016) Antiangiogenic effect of bevacizumab: Application of arterial spin-labeling perfusion MR imaging in a rat glioblastoma model. Am J Neuroradiol (Internet) 37:1650–1656. https://doi.org/10.3174/ajnr.A4800
Wang L, Wei L, Wang J, Li N, Gao Y, Ma H, Qu X, Zhang M (2020) Evaluation of perfusion MRI value for tumor progression assessment after glioma radiotherapy: a systematic review and meta-analysis. Medicine (Baltimore) 99:e23766. https://doi.org/10.1097/MD.0000000000023766
O’Connor JPB, Aboagye EO, Adams JE, Aerts HJWL, Barrington SF, Beer AJ, Boellaard R, Bohndiek SE, Brady M, Brown G, Buckley DL, Chenevert TL, Clarke LP et al (2017) Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol (Internet, Nature Publishing Group). 14:169–186. https://doi.org/10.1038/nrclinonc.2016.162
Petersen ET, Mouridsen K, Golay X (2010) The QUASAR reproducibility study, Part II: Results from a multi-center arterial spin labeling test-retest study. Neuroimage (Internet, Elsevier Inc). 49:104–113. https://doi.org/10.1016/j.neuroimage.2009.07.068
Chen Y, Wang DJJ, Detre JA (2011) Test-retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging (Internet) 33:940–949. https://doi.org/10.1002/jmri.22345
Zhou L, Wang Y, Pinho MC, Pan E, Xi Y, Maldjian JA, Madhuranthakam AJ (2020) Intrasession reliability of arterial spin-labeled MRI-measured noncontrast perfusion in glioblastoma at 3 T. Tomography (Internet) 6:139–147. https://doi.org/10.18383/j.tom.2020.00010
Warmuth C, Günther M, Zimmer C (2003) Quantification of blood flow in brain tumors: Comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging. Radiology 228(523):532. https://doi.org/10.1148/radiol.2282020409
Cebeci H, Aydin O, Ozturk-Isik E, Gumus C, Inecikli F, Bekar A, Kocaeli H, Hakyemez B (2014) Assesment of perfusion in glial tumors with arterial spin labeling; comparison with dynamic susceptibility contrast method. Eur J Radiol (Internet, Elsevier Ireland Ltd) 83:1914–1919. https://doi.org/10.1016/j.ejrad.2014.07.002
Figueiredo PM, Clare S, Jezzard P (2005) Quantitative perfusion measurements using pulsed arterial spin labeling: effects of large region-of-interest analysis. J Magn Reson Imaging (Internet) 21:676–682. https://doi.org/10.1002/jmri.20329
Fallatah S (2016) Perfusion imaging in brain tumours. UCL (University College London), London
Melbourne A, Toussaint N, Owen D, Simpson I, Anthopoulos T, De Vita E, Atkinson D, Ourselin S (2016) NiftyFit: a software package for multi-parametric model-fitting of 4D magnetic resonance imaging data. Neuroinformatics 14:319–337. https://doi.org/10.1007/s12021-016-9297-6
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage (Internet) 17:825–841. https://doi.org/10.1006/nimg.2002.1132
Mignani S, Rosa R (1995) The moving block bootstrap to assess the accuracy of statistical estimates in Ising model simulations. Comput Phys Commun 92:203–213. https://doi.org/10.1016/0010-4655(95)00114-7
Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez-Garcia L, Lu H, Macintosh BJ, Parkes LM, Smits M, Van Osch MJP, Wang DJJ, Wong EC et al (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116. https://doi.org/10.1002/mrm.25197
Wansapura JP, Holland SK, Dunn RS, Ball WS (1999) NMR relaxation times in the human brain at 3.0 Tesla. J Magn Reson Imaging 9:531–538. https://doi.org/10.1002/(SICI)1522-2586(199904)9:4<531::AID-JMRI4>3.0.CO;2-L
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015
Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011) A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage (Internet) 56:907–922. https://doi.org/10.1016/j.neuroimage.2011.02.046
Modat M, Ridgway GR, Taylor ZA, Lehmann M, Barnes J, Hawkes DJ, Fox NC, Ourselin S (2010) Fast free-form deformation using graphics processing units. Comput Methods Programs Biomed 98:278–284. https://doi.org/10.1016/j.cmpb.2009.09.002
NEMA (2014) NEMA Standards Publication MS 1-2001: Determination of signal-to-noise ratio (SNR) in diagnostic magnetic resonance imaging. Natl Electr Manuf Assoc (Internet) 2008:21. https://www.nema.org/Standards/Pages/Determination-of-Signal-to-Noise-Ratio-in-Diagnostic-Magnetic-Resonance-Imaging.aspx
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med (Internet, Elsevier B.V.) 15:155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Bland M (2006) How do I calculate a within-subject coefficient of variation (Internet). https://www-users.york.ac.uk/~mb55/meas/cv.htm
Vaz S, Falkmer T, Passmore AE, Parsons R, Andreou P (2013) The case for using the repeatability coefficient when calculating test-retest reliability. PLoS ONE 8:1–7. https://doi.org/10.1371/journal.pone.0073990
Schuck P, Zwingmann C (2003) The ‘smallest real difference’ as a measure of sensitivity to change: a critical analysis. Int J Rehabil Res 26:85–91. https://doi.org/10.1097/01.mrr.0000070759.63544.65
Wang Y, Saykin AJ, Pfeuffer J, Lin C, Mosier KM, Shen L, Kim S, Hutchins GD (2011) Regional reproducibility of pulsed arterial spin labeling perfusion imaging at 3T. Neuroimage (Internet; Elsevier Inc.) 54:1188–1195. https://doi.org/10.1016/j.neuroimage.2010.08.043
Mutsaerts HJMM, Steketee RME, Heijtel DFR, Kuijer JPA, Van Osch MJP, Majoie CBLM, Smits M, Nederveen AJ (2015) Inter-vendor reproducibility of pseudo-continuous arterial spin labeling at 3 Tesla. PLoS ONE 2014:9. https://doi.org/10.1371/journal.pone.0104108
Aslan S, Lu H (2010) On the sensitivity of ASL MRI in detecting regional differences in cerebral blood flow. Magn Reson Imaging (Internet; Elsevier Inc) 28:928–935. https://doi.org/10.1016/j.mri.2010.03.037
Mezue M, Segerdahl AR, Okell TW, Chappell MA, Kelly ME, Tracey I (2014) Optimization and reliability of multiple postlabeling delay pseudo-continuous arterial spin labeling during rest and stimulus-induced functional task activation. J Cereb Blood Flow Metab 34:1919–1927. https://doi.org/10.1038/jcbfm.2014.163
Raunig DL, McShane LM, Pennello G, Gatsonis C, Carson PL, Voyvodic JT, Wahl RL, Kurland BF, Schwarz AJ, Gönen M, Zahlmann G, Kondratovich MV, O’Donnell K et al (2015) Quantitative imaging biomarkers: a review of statistical methods for technical performance assessment. Stat Methods Med Res (Internet) 24:27–67. https://doi.org/10.1177/0962280214537344
Sokolska M, Bainbridge A, Rojas-Villabona A, Golay X, Thomas DL (2019) Effect of labelling plane angulation and position on labelling efficiency and cerebral blood flow quantification in pseudo-continuous arterial spin labelling. Magn Reson Imaging (Internet; Elsevier) 59:61–67. https://doi.org/10.1016/j.mri.2019.02.007
Robertson AD, Matta G, Basile VS, Black SE, Macgowan CK, Detre JA, MacIntosh BJ (2017) Temporal and spatial variances in arterial spin-labeling are inversely related to large-artery blood velocity. Am J Neuroradiol 38:1555–1561. https://doi.org/10.3174/ajnr.A5257
Yang XS, Zhao B, Wang G, Xiang J, Xu S, Liu Y, Zhao P, Pfeuffer J, Qian T (2016) Improving the grading accuracy of astrocytic neoplasms noninvasively by combining timing information with cerebral blood flow: a multi-Ti arterial spin-labeling MR imaging study. Am J Neuroradiol (Internet) 37:2209–2216. https://doi.org/10.3174/ajnr.A4907
Acknowledgements
AFA is funded by Taibah University from the Saudi Arabia Government. DLT was supported by the UCL Leonard Wolfson Experimental Neurology Centre (PR/ylr/18575), and the Wellcome Trust (Centre award 539208). EDV is funded by the Wellcome Engineering and Physical Sciences Research Council (EPSRC) Centre for Medical Engineering at Kings College London [WT 203148/Z/16/Z].SB is funded by National Institute for Health Research to UCLH Biomedical research centre (Grant: BRC399/NS/RB/101410). XG received funding by the European Union’s Horizon 2020 research and innovation programme, Grant/Award Number: 667510. This work received funding from the UCLH NIHR Biomedical Research Centre (DLT, SB, XG).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Study conception and design were fulfilled by XG, SB and AFA. Data collection were performed by DLT and EDV. Analysing and interpretation of the data were performed by AFA and JP-G. The first draft of the manuscript was written by AFA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article. XG is founder, shareholder and CEO of Gold Standard Phantoms, a UCL spinout company producing, among others, ASL phantoms.
Ethical approval
All participants provided signed informed consent, and the study was approved by the Queen Square Research Ethics committee (IRB).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alsaedi, A.F., Thomas, D.L., De Vita, E. et al. Repeatability of perfusion measurements in adult gliomas using pulsed and pseudo-continuous arterial spin labelling MRI. Magn Reson Mater Phy 35, 113–125 (2022). https://doi.org/10.1007/s10334-021-00975-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-021-00975-4