Abstract
Objective
Using non-invasive magnetic resonance (MR) techniques and a histological approach, we assessed the outcomes of perinatal exposure at a low dose of 3,3′-DCBPA (2-chloro-4-[1-(3-chloro-4-hydroxyphenyl)-1-methylethyl]phenol) and/or 3,5-DCBPA (2,6-dichloro-4-[1-(4-hydroxyphenyl)-1-methylethyl]phenol) on mice livers.
Materials and methods
Fertilized female Swiss mice were injected intraperitoneally during gestation and lactation with either vehicle control, 20 μg/kg/day of BPA, 3,5-DCBPA, 3,3′-DCBPA or a mixture (mix-DCBPA). Complementary methods were used to evaluate, in male and female pups, (1) liver structure by texture analysis of images obtained through MR imaging (MRI) and histology, (2) hepatic lipid composition through in vivo 1H MR spectroscopy (1H MRS).
Results
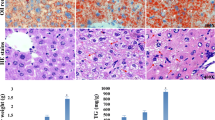
Principal component analysis of texture parameters showed no structural modification of the liver with BPA and DCBPA treatments. Accordingly, no hepatic microvesicular steatosis was observed through hematoxylin–eosin staining. Compared to control, MRS revealed no difference in lipid composition for BPA, 3,5-DCBPA or 3,3′-DCBPA groups. However, MRS detected a significant increase in the mix-DCBPA groups for the saturated component of fatty acids (FA), total unsaturated FA bond index and polyunsaturated FA bond index.
Conclusion
Prior to any structural changes, polyunsaturated fatty acids significantly increased in young male and female mice exposed perinatally at a low dose to a mixture of dichlorinated BPA.







Similar content being viewed by others
References
Staples CA, Dorn PB, Klecka GM, O’Block ST, Harris LR (1998) A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 36(10):2149–2173
Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM (2009) Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 30(1):75–95
Muhamad MS, Salim MR, Lau WJ, Yusop Z (2016) A review on bisphenol A occurrences, health effects and treatment process via membrane technology for drinking water. Environ Sci Pollut Res 23(12):11549–11567
Kang J-H, Kondo F, Katayama Y (2006) Human exposure to bisphenol A. Toxicology 226(2–3):79–89
Kuruto-Niwa R, Terao Y, Nozawa R (2002) Identification of estrogenic activity of chlorinated bisphenol A using a GFP expression system. Environ Toxicol Pharmacol 12(1):27–35
Takemura H, Ma J, Sayama K, Terao Y, Zhu BT, Shimoi K (2005) In vitro and in vivo estrogenic activity of chlorinated derivatives of bisphenol A. Toxicology 207(2):215–221
Hu J-Y, Aizawa T, Ookubo S (2002) Products of aqueous chlorination of bisphenol A and their estrogenic activity. Environ Sci Technol 36(9):1980–1987
Yamamoto T, Yasuhara A (2002) Chlorination of bisphenol A in aqueous media: formation of chlorinated bisphenol A congeners and degradation to chlorinated phenolic compounds. Chemosphere 46(8):1215–1223
Fukazawa H, Hoshino K, Shiozawa T, Matsushita H, Terao Y (2001) Identification and quantification of chlorinated bisphenol A in wastewater from wastepaper recycling plants. Chemosphere 44(5):973–979
Fan Z, Hu J, An W, Yang M (2013) Detection and occurrence of chlorinated byproducts of bisphenol A, nonylphenol, and estrogens in drinking water of China: comparison to the parent compounds. Environ Sci Technol 47(19):10841–10850
Zhou Y, Chen M, Zhao F, Mu D, Zhang Z, Hu J (2015) Ubiquitous occurrence of chlorinated byproducts of bisphenol A and nonylphenol in bleached food contacting papers and their implications for human exposure. Environ Sci Technol 49(12):7218–7226
Andra SS, Charisiadis P, Arora M, van Vliet-Ostaptchouk JV, Makris KC (2015) Biomonitoring of human exposures to chlorinated derivatives and structural analogs of bisphenol A. Environ Int 85:352–379
Fernandez MF et al (2007) Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol Elmsford N 24(2):259–264
Jiménez-Díaz I et al (2010) Determination of Bisphenol A and its chlorinated derivatives in placental tissue samples by liquid chromatography–tandem mass spectrometry. J Chromatogr B 878(32):3363–3369
Cariot A, Dupuis A, Albouy-Llaty M, Legube B, Rabouan S, Migeot V (2012) Reliable quantification of bisphenol A and its chlorinated derivatives in human breast milk using UPLC–MS/MS method. Talanta 100:175–182
Migeot V, Dupuis A, Cariot A, Albouy-Llaty M, Pierre F, Rabouan S (2013) Bisphenol A and its chlorinated derivatives in human colostrum. Environ Sci Technol 47(23):13791–13797
Liao C, Kannan K (2012) Determination of free and conjugated forms of bisphenol A in human urine and serum by liquid chromatography-tandem mass spectrometry. Environ Sci Technol 46(9):5003–5009
Venisse N et al (2014) Reliable quantification of bisphenol A and its chlorinated derivatives in human urine using UPLC–MS/MS method. Talanta 125:284–292
Chen M et al (2016) Occurrence and maternal transfer of chlorinated bisphenol A and nonylphenol in pregnant women and their matching embryos. Environ Sci Technol 50(2):970–977
vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA (2012) The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol 354(1–2):74–84
Chevalier N, Fénichel P (2015) Endocrine disruptors: new players in the pathophysiology of type 2 diabetes? Diabetes Metab 41(2):107–115
Alonso-Magdalena P, Quesada I, Nadal A (2015) Prenatal exposure to BPA and offspring outcomes: the diabesogenic behavior of BPA. Dose-Resp 13(2):1559325815590395
Jiang Y et al (2014) Mitochondrial dysfunction in early life resulted from perinatal bisphenol A exposure contributes to hepatic steatosis in rat offspring. Toxicol Lett 228(2):85–92
Marmugi A et al (2012) Low doses of bisphenol a induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology 55(2):395–407
Begriche K, Igoudjil A, Pessayre D, Fromenty B (2006) Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion 6(1):1–28
Yu H et al (2015) Utility of texture analysis for quantifying hepatic fibrosis on proton density MRI: hepatic fibrosis on proton density MRI. J Magn Reson Imaging 42(5):1259–1265
Yokoo T et al (2015) Evaluation of liver fibrosis using texture analysis on combined-contrast-enhanced magnetic resonance images at 3.0 T. Biomed Res Int 2015:1–12
Wu Z et al (2015) Hepatitis C related chronic liver cirrhosis: feasibility of texture analysis of mr images for classification of fibrosis stage and necroinflammatory activity grade. PLoS One 10(3):e0118297
Wang D, Li Y (2015) 1H magnetic resonance spectroscopy predicts hepatocellular carcinoma in a subset of patients with liver cirrhosis: a randomized trial. Medicine (Baltimore) 94(27):e1066
Zhang L, Zhao X, Ouyang H, Wang S, Zhou C (2016) Diagnostic value of 3.0T 1H MRS with choline-containing compounds ratio (∆CCC) in primary malignant hepatic tumors. Cancer Imaging 16(1):25
Bohte AE, van Werven JR, Bipat S, Stoker J (2011) The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 21(1):87–97
Ramamonjisoa N et al (2013) In vivo hepatic lipid quantification using MRS at 7 Tesla in a mouse model of glycogen storage disease type 1a. J Lipid Res 54(7):2010–2022
Cobbold JFL et al (2010) Hepatic lipid profiling in chronic hepatitis C: an in vitro and in vivo proton magnetic resonance spectroscopy study. J Hepatol 52(1):16–24
Herlidou S, Rolland Y, Bansard JY, Le Rumeur E, de Certaines JD (1999) Comparison of automated and visual texture analysis in MRI: characterization of normal and diseased skeletal muscle. Magn Reson Imaging 17(9):1393–1397
Szczypiński PM, Strzelecki M, Materka A, Klepaczko A (2009) MaZda—a software package for image texture analysis. Comput Methods Progr Biomed 94(1):66–76
Corbin IR, Furth EE, Pickup S, Siegelman ES, Delikatny EJ (2009) In vivo assessment of hepatic triglycerides in murine non-alcoholic fatty liver disease using magnetic resonance spectroscopy. Biochim Biophys Acta Bba Mol Cell Biol Lipids 1791(8):757–763
van Werven JR, Marsman HA, Nederveen AJ, ten Kate FJ, van Gulik TM, Stoker J (2012) Hepatic lipid composition analysis using 3.0-T MR spectroscopy in a steatotic rat model. Magn Reson Imaging 30(1):112–121
Tandra S et al (2011) Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J Hepatol 55(3):654–659
Barker DJP (1998) In utero programming of chronic disease. Clin Sci 95(2):115
Zalko D et al (2003) Biotransformations of bisphenol A in a mammalian model: answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice. Environ Health Perspect 111(3):309–319
Pritchett JJ, Kuester RK, Sipes IG (2002) Metabolism of bisphenol A in primary cultured hepatocytes from mice, rats, and humans. Drug Metab Dispos Biol Fate Chem 30(11):1180–1185
Taylor JA, Welshons WV, Vom Saal FS (2008) No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24 h after administration in neonatal female mice. Reprod Toxicol 25(2):169–176
Patel BB, Di Iorio M, Chalifour LE (2014) Metabolic response to chronic bisphenol A exposure in C57bl/6n mice. Toxicol Rep 1:522–532
Angle BM et al (2013) Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol Elmsford N 42:256–268
Alonso-Magdalena P et al (2010) Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect 118(9):1243–1250
Rubin BS, Soto AM (2009) Bisphenol A: perinatal exposure and body weight. Mol Cell Endocrinol 304(1–2):55–62
Strakovsky RS et al (2015) Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol Appl Pharmacol 284(2):101–112
Galyon KD, Farshidi F, Han G, Ross MG, Desai M, Jellyman JK (2016) Maternal bisphenol A exposure alters rat offspring hepatic and skeletal muscle insulin signaling protein abundance. Am J Obstet Gynecol 216(3):290.e1–e9
Shibata N, Matsumoto J, Nakada K, Yuasa A, Yokota H (2002) Male-specific suppression of hepatic microsomal UDP-glucuronosyl transferase activities toward sex hormones in the adult male rat administered bisphenol A. Biochem J 368(3):783–788
Araya J et al (2004) Increase in long-chain polyunsaturated fatty acid n − 6/n − 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci 106(6):635–643
Videla LA, Rodrigo R, Araya J, Poniachik J (2004) Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic Biol Med 37(9):1499–1507
Gajdošík M et al (2014) In vivo relaxation behavior of liver compounds at 7 tesla, measured by single-voxel proton MR spectroscopy: relaxation of liver compounds at 7 T. J Magn Reson Imaging 40(6):1365–1374
Williams E, Hamilton JA, Jain MK, Allerhand A, Cordes EH, Ochs S (1973) Natural abundance carbon-13 nuclear magnetic resonance spectra of the canine sciatic nerve. Science 181(4102):869–871
Chen M et al (2012) Bisphenol A alters n-6 fatty acid composition and decreases antioxidant enzyme levels in rat testes: a LC-QTOF-Based metabolomics study. PLoS One 7(9):e44754
Grasselli E et al (2013) Direct effects of Bisphenol A on lipid homeostasis in rat hepatoma cells. Chemosphere 91(8):1123–1129
Fukazawa H et al (2002) Formation of chlorinated derivatives of bisphenol A in waste paper recycling plants and their estrogenic activities. J Heal Sci 48(3):242–249
Viñas R, Goldblum RM, Watson CS (2013) Rapid estrogenic signaling activities of the modified (chlorinated, sulfonated, and glucuronidated) endocrine disruptor bisphenol A. Endocr Disrupt 1(1):e25411
Riu A et al (2011) Characterization of novel ligands of ER, Er, and PPAR: the case of halogenated bisphenol A and their conjugated metabolites. Toxicol Sci 122(2):372–382
Acknowledgements
We gratefully acknowledge Laurence Turi for her technical support in animal care and Alba Casas Mora who drew the molecules in Fig. 1. The authors declare that there are no conflicts of interest.
Funding
This work was supported by the Region Centre-Val de Loire (France) (Grant number: no. 201400093609 to SM) and Ligue contre le cancer (France) (to ND).
Author information
Authors and Affiliations
Contributions
DE participated in the study design, contributed to data acquisition, analysis, interpretation, and drafted the manuscript. AC was involved in study design, data acquisition, analysis and revising the manuscript. WM supervised the data acquisition, analysis, interpretation and revising the manuscript. MM was involved in interpretation and revising the manuscript. ND was involved in study design, data collection, funding application and revising the manuscript. SM supervised the data acquisition, analysis, interpretation and was involved in funding application and revising the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the French Ministry of Agriculture and Forests (Animal Health and Protection Veterinary Service). The protocol has been approved by the local ethics committee “COMETHEA no. 84” (no. 02.160).
Rights and permissions
About this article
Cite this article
El Hamrani, D., Chepied, A., Même, W. et al. Gestational and lactational exposure to dichlorinated bisphenol A induces early alterations of hepatic lipid composition in mice. Magn Reson Mater Phy 31, 565–576 (2018). https://doi.org/10.1007/s10334-018-0679-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-018-0679-7