Abstract
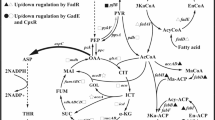
Fine tuning of the key enzymes to moderate rather than high expression levels could overproduce the desired metabolic products without inhibiting cell growth. The aims of this investigation were to regulate rates of lactate production and cell growth in recombinant Escherichia coli through promoter engineering and to evaluate the transcriptional function of the upstream region of ldhA (encoding fermentative lactate dehydrogenase in E. coli). Twelve ldhA genes with sequentially shortened chromosomal upstream regions were cloned in an ldhA deletion, E. coli CICIM B0013-080C (ack-pta pps pflB dld poxB adhE frdA ldhA). The varied ldhA upstream regions were further analyzed using program NNPP2.2 (Neural Network Promoter Prediction 2.2) to predict the possible promoter regions. Two-phase fermentations (aerobic growth and oxygen-limited production) of these strains showed that shortening the ldhA upstream sequence from 291 to 106 bp successively reduced aerobic lactate synthesis and the inhibition effect on cell growth during the first phase. Simultaneously, oxygen-limited lactate productivity was increased during the second phase. The putative promoter downstream of the −96 site of ldhA could function as a transcriptional promoter or regulator. B0013-080C/pTH-rrnB-ldhA8, with the 72-bp upstream segment of ldhA, could be grown at a high rate and achieve a high oxygen-limited lactate productivity of 1.09 g g−1 h−1. No transcriptional promoting region was apparent downstream of the −61 site of ldhA. We identified the latent transcription regions in the ldhA upstream sequence, which will help to understand regulation of ldhA expression.

Similar content being viewed by others
References
Alper H, Fischer C, Nevoigt E, Stephanopoulos G (2005) Tuning genetic control through promoter engineering. Proc Natl Acad Sci USA 102:12678–12683
Bunch PK, Mat-Jan F, Lee N, Clark DP (1997) The ldhA gene encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology 143:187–195
Burden S, Lin YX, Zhang R (2005) Improving promoter prediction for the NNPP2. 2 algorithm: a case study using Escherichia coli DNA sequences. Bioinformatics 21:601–607
Demeler B, Zhou G (1991) Neural network optimization for E. coli promoter prediction. Nucleic Acids Res 19:1593–1599
Hashimoto-Gotoh T, Yamaguchi M, Yasojima K, Tsujimura A, Wakabayashi Y, Watanabe Y (2000) A set of temperature sensitive-replication/-segregation and temperature resistant plasmid vectors with different copy numbers and in an isogenic background (chloramphenicol, kanamycin, lacZ, repA, par, polA). Gene 241:185–191
Huerta AM, Collado-Vides J (2003) Sigma70 promoters in Escherichia coli: specific transcription in dense regions of overlapping promoter-like signals. J Mol Biol 333:261–278
Jensen PR, Hammer K (1998) The sequence of spacers between the consensus sequences modulates the strength of prokaryotic promoters. Appl Environ Microbiol 64:82–87
Jiang GR, Nikolova S, Clark DP (2001) Regulation of the ldhA gene, encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology 147:2437–2446
Jin YS, Ni H, Laplaza JM, Jeffries TW (2003) Optimal growth and ethanol production from xylose by recombinant Saccharomyces cerevisiae require moderate d-xylulokinase activity. Appl Environ Microbiol 69:495–503
Kalate RN, Tambe SS, Kulkarni BD (2003) Artificial neural networks for prediction of mycobacterial promoter sequences. Comput Biol Chem 27:555–564
Liu R, States DJ (2002) Consensus promoter identification in the human genome utilizing expressed gene markers and gene modeling. Genome Res 12:462–469
Nevoigt E, Fischer C, Mucha O, Matthus F, Stahl U, Stephanopoulos G (2007) Engineering promoter regulation. Biotechnol Bioeng 96:550–558
Nevoigt E, Kohnke J, Fischer CR, Alper H, Stahl U, Stephanopoulos G (2006) Engineering of promoter replacement cassettes for fine-tuning of gene expression in Saccharomyces cerevisiae. Appl Environ Microbiol 72:5266–5273
Reitzer LJ, Bueno R, Cheng WD, Abrams SA, Rothstein DM, Hunt TP, Tyler B, Magasanik B (1987) Mutations that create new promoters suppress the sigma 54 dependence of glnA transcription in Escherichia coli. J Bacteriol 169:4279–4284
Zhao K, Liu M, Burgess RR (2005) The global transcriptional response of Escherichia coli to induced σ32 protein involves σ32 regulon activation followed by inactivation and degradation of σ32 in vivo. J Biol Chem 280:17758–17768
Zhou L, Zuo ZR, Chen XZ, Niu DD, Tian KM, Prior BA, Shen W, Shi GY, Singh S, Wang ZX (2011) Evaluation of genetic manipulation strategies on d-lactate production by Escherichia coli. Curr Microbiol 62:981–989
Acknowledgments
This work was partly funded by the Sino-South Africa Cooperation Program 2009DFA31300 and the National Natural Science Foundation of China no. 21006039.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10295_2012_1116_MOESM2_ESM.tif
Representative experiments (conducted in duplicate) showing the production of lactate during the two phase fermentation by B0013-080C/pTH-rrnB-ldhA0 to B0013-080C/pTH-rrnB-ldhA11 as well as B0013-070 and B0013-080C. The dotted lines (marked by 0 to 13) indicate the time when the cultures of the strains were shifted from the aerobic growth to the oxygen-limited production phase (TIFF 1075 kb)
Rights and permissions
About this article
Cite this article
Zhou, L., Shen, W., Niu, DD. et al. Fine tuning the transcription of ldhA for d-lactate production. J Ind Microbiol Biotechnol 39, 1209–1217 (2012). https://doi.org/10.1007/s10295-012-1116-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1116-y