Abstract
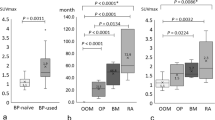
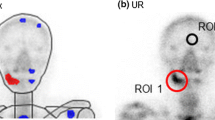
We examined the changes in the bone metabolism of the jaw in response to BP treatment, and we used bone SPECT-CT to analyze the site-specific bone metabolism between the jaw and other sites of bone. We compared the changes in the bone metabolism of each part of bone in response to BP treatment by performing a quantitative analysis of bone scintigraphy images between patients treated with low-dose BP for osteoporosis (LBP group; n = 17), those treated with high-dose BP for metastatic bone tumor (HBP group; n = 11), and patients with other oral disease who required bone scintigraphy, with no history of BP treatment (control group; n = 40). The study endpoint was the mean standardized uptake value (SUV) of the uptake of Tc-99 m methylene diphosphonate (MDP) in each group. The mean SUVs of the HBP group were significantly lower at the axial bone of the cervical vertebra, thoracic vertebra, sternum, and rib compared to those of the LBP and control groups. The LBP group's mean SUV was significantly higher at the temporal bone, the anodontia part of the alveolar bone in maxilla, the vital teeth part of alveolar bone in the mandible, and the temporomandibular joint. There was no significant difference among the three groups at the mandibular angle and mandibular ramus. Our analyses revealed that the bone metabolism of the jaw and temporal bone in the BP-treated patients was enhanced, and no suppression of bone remodeling in the jaw by BP was observed.






Similar content being viewed by others
References
Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–56. https://doi.org/10.1016/j.joms.2014.04.031.
Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115–7.
Migliorati CA. Bisphosphanates and oral cavity avascular bone necrosis. J Clin Oncol. 2003;21(22):4253–4. https://doi.org/10.1200/JCO.2003.99.132.
Kaneta T, Ogawa M, Daisaki H, Nawata S, Yoshida K, Inoue T. SUV measurement of normal vertebrae using SPECT/CT with Tc-99m methylene diphosphonate. Am J Nuclear Med Mol Imag. 2016;6(5):262–8.
Win AZ, Aparici CM. Normal SUV values measured from NaF18-PET/CT bone scan studies. PloS ONE. 2014;9(9):e108429. https://doi.org/10.1371/journal.pone.0108429.
Ohbayashi Y, Nakai F, Iwasaki A, Ogawa T, Yamamoto Y, Nishiyama Y, et al. The utility of bone scintigraphy in the assessment of mandibular metabolism during long-term bisphosphonate administration. Odontology. 2017;105(3):382–90. https://doi.org/10.1007/s10266-016-0279-9.
Thomas C, Spanidis M, Engel C, Roos FC, Frees S, Neisius A, et al. Bone scintigraphy predicts bisphosphonate-induced osteonecrosis of the jaw (BRONJ) in patients with metastatic castration-resistant prostate cancer (mCRPC). Clin Oral Investig. 2016;20(4):753–8. https://doi.org/10.1007/s00784-015-1563-8.
O'Ryan FS, Khoury S, Liao W, Han MM, Hui RL, Baer D, et al. Intravenous bisphosphonate-related osteonecrosis of the jaw: bone scintigraphy as an early indicator. J Oral Maxillofac. 2009;67(7):1363–72. https://doi.org/10.1016/j.joms.2009.03.005.
Hong CM, Ahn BC, Choi SY, Kim DH, Lee SW, Kwon TG, et al. Implications of three-phase bone scintigraphy for the diagnosis of bisphosphonate-related osteonecrosis of the jaw. Nuclear Med Mol Imag. 2012;46(3):162–8. https://doi.org/10.1007/s13139-012-0144-x.
Allen MR. Medication-related osteonecrosis of the jaw: basic and translational science updates. Oral Maxillofac Surg Clin N Am. 2015;27(4):497–508. https://doi.org/10.1016/j.coms.2015.06.002.
Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144(10):753–61.
Reid IR, Bolland MJ, Grey AB. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007;41(3):318–20. https://doi.org/10.1016/j.bone.2007.04.196.
Khan RA, Hughes S, Lavender P, Leon M, Spyrou N. Autoradiography of technetium-labelled diphosphonate in rat bone. J Bone Joint Surg Br. 1979;61(2):221–4.
Francis MD, Ferguson DL, Tofe AJ, Bevan JA, Michaels SE. Comparative evaluation of three diphosphonates: in vitro adsorption (C-14 labeled) and in vivo osteogenic uptake (Tc-99m complexed). J Nucl Med. 1980;21(12):1185–9.
Christensen SB, Arnold CC. Distribution of 99mTc-phosphate compounds in osteoarthritic femoral heads. J Bone Joint Surg Am. 1980;62(1):90–6.
Kanishi D. 99mTc-MDP accumulation mechanisms in bone. Oral Surg Oral Med Oral Pathol. 1993;75(2):239–46.
Christensen SB, Krogsgaard OW. Localization of Tc-99m MDP in epiphyseal growth plates of rats. J Nucl Med. 1981;22(3):237–45.
Savelkoul TJ, Visser WJ, Oldenburg SJ, Duursma SA. A micro-autoradiographical study of the localization of 99mTc(Sn)-MDP and 99mTc-MDP in undecalcified bone sections. Eur J Nucl Med. 1986;11(11):459–62.
Budd RS, Hodgson GS, Hare WS. The relation of radionuclide uptake by bone to the rate of calcium mineralization II: patient studies using 99Tcm-MDP. Br J Radiol. 1989;62(736):318–20. https://doi.org/10.1259/0007-1285-62-736-318.
Lausten GS, Christensen SB. Distribution of 99mTc-phosphate compounds in osteonecrotic femoral heads. Acta Orthop Scand. 1989;60(4):419–23.
Puri T, Frost ML, Curran KM, Siddique M, Moore AE, Cook GJ, et al. Differences in regional bone metabolism at the spine and hip: a quantitative study using (18)F-fluoride positron emission tomography. Osteop Int. 2013;24(2):633–9. https://doi.org/10.1007/s00198-012-2006-x.
Cheng C, Heiss C, Dimitrakopoulou-Strauss A, Govindarajan P, Schlewitz G, Pan L, et al. Evaluation of bone remodeling with (18)F-fluoride and correlation with the glucose metabolism measured by (18)F-FDG in lumbar spine with time in an experimental nude rat model with osteoporosis using dynamic PET-CT. Am J Nuclear Med Mol Imag. 2013;3(2):118–28.
Frost ML, Siddique M, Blake GM, Moore AE, Schleyer PJ, Dunn JT, et al. Differential effects of teriparatide on regional bone formation using (18)F-fluoride positron emission tomography. J Bone Miner Res. 2011;26(5):1002–111. https://doi.org/10.1002/jbmr.305.
Kobayashi N, Inaba Y, Tateishi U, Yukizawa Y, Ike H, Inoue T, et al. New application of 18F-fluoride PET for the detection of bone remodelling in early-stage osteoarthritis of the hip. Clin Nuclear Med. 2013;38(10):e379–e383383. https://doi.org/10.1097/RLU.0b013e31828d30c0.
Austin AG, Raynor WY, Reilly CC, Zadeh MZ, Werner TJ, Zhuang H, et al. Evolving role of MR imaging and PET in assessing osteoporosis. PET Clin. 2019;14(1):31–41. https://doi.org/10.1016/j.cpet.2018.08.007.
Suenaga H, Yokoyama M, Yamaguchi K, Sasaki K. Bone metabolism of residual ridge beneath the denture base of an RPD observed using NaF-PET/CT. J Prosthodont Res. 2012;56(1):42–6. https://doi.org/10.1016/j.jpor.2011.04.002.
Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endoc Rev. 2000;21(2):115–37. https://doi.org/10.1210/edrv.21.2.0395.
Civitelli R, Gonnelli S, Zacchei F, Bigazzi S, Vattimo A, Avioli LV, et al. Bone turnover in postmenopausal osteoporosis effect of calcitonin treatment. J Clin Invest. 1988;82(4):1268–74. https://doi.org/10.1172/JCI113725.
Caniggia A, Vattimo A. Kinetics of 99mtechnetium-tin-methylene-diphosphonate in normal subjects and pathological conditions: a simple index of bone metabolism. Calcif Tissue Int. 1980;30(1):5–13.
Davie MW, Britton JM, Haddaway M, McCall IW. 99mTc-MDP retention in osteoporosis: relationship to other indices of bone cell activity and response to calcium and vitamin D therapy. Eur J Nucl Med. 1987;13(9):462–6.
Frost ML, Fogelman I, Blake GM, Marsden PK, Cook G Jr. Dissociation between global markers of bone formation and direct measurement of spinal bone formation in osteoporosis. J Bone Min Res. 2004;19(11):1797–804. https://doi.org/10.1359/JBMR.040818.
Israel O, Lubushitzky R, Frenkel A, Iosilevsky G, Bettman L, Gips S, et al. Bone turnover in cortical and trabecular bone in normal women and in women with osteoporosis. J Nucl Med. 1994;35(7):1155–8.
Carnevale V, Dicembrino F, Frusciante V, Chiodini I, Minisola S, Scillitani A. Different patterns of global and regional skeletal uptake of 99mTc-methylene diphosphonate with age: relevance to the pathogenesis of bone loss. J Nucl Med. 2000;41(9):1478–83.
Uchida K, Nakajima H, Miyazaki T, Yayama T, Kawahara H, Kobayashi S, et al. Effects of alendronate on bone metabolism in glucocorticoid-induced osteoporosis measured by 18F-fluoride PET: a prospective study. J Nucl Med. 2009;50(11):1808–14. https://doi.org/10.2967/jnumed.109.062570.
Suh MS, Lee WW, Kim YK, Yun PY, Kim SE. Maximum Standardized Uptake Value of (99m)Tc Hydroxymethylene diphosphonate SPECT/CT for the evaluation of temporomandibular joint disorder. Radiology. 2016;280(3):890–6. https://doi.org/10.1148/radiol.2016152294.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee [the Kagawa University Ethical Committee (H24–#106)] and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakai, F., Ohbayashi, Y., Nakai, Y. et al. Bone metabolism of the jaw in response to bisphosphonate: a quantitative analysis of bone scintigraphy images. Odontology 108, 653–660 (2020). https://doi.org/10.1007/s10266-020-00503-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-020-00503-1