Abstract
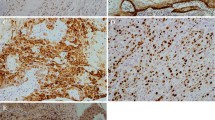
To evaluate erythropoietin-producing hepatocellular carcinoma (Eph)/Eph receptor-interaction protein (ephrin) expression in oral squamous cell carcinoma (OSCC) and oral epithelial precursor lesions (OEPLs), EphA2, EphB4, and ephrinB2 were examined and compared with microvessel density (MVD) and lymphatic vessel density (LVD). Samples from 73 OSCC and 43 OEPLs patients were immunohistochemically analyzed with antibodies against EphA2, EphB4, ephrinB2, CD34, and D2-40. Results were compared with clinicopathological findings. Immunohistochemical reactivity for EphA2, EphB4, and ephrinB2 was detected in epithelial cells and some stromal vascular cells in OEPLs and OSCC, proportionately with the level of malignancy. The number of blood vessel endothelial cells stained with CD34 and lymphatic vessel endothelial cells stained with D2-40 was increased in OEPLs and OSCC. In OSCC, ephrinB2 and EphB4 exhibited significant correlation with recurrence and invasion depth, respectively. MVD was significantly lower in slight lymphocytic reaction than in prominent stromal reaction. Association was found between LVD and T classification, postoperative metastasis, survival, mode of invasion, and invasion depth. Expression of EphA2, EphB4, ephrinB2, MVD, and LVD might be associated with malignant potential of the oral epithelium. Angiogenesis and lymphangiogenesis appear to be related to progression of potentially malignant oral lesions.

Similar content being viewed by others
References
Gale N, Hille J, Jordan RC, Nadal A, Williams MD. Tumors of the oral cavity and mobile tongue. WHO classification of head and neck tumours. Lyon: IARC Press; 2017. p. 91–3.
Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–16.
Kouketsu A, Sato I, Abe S, Oikawa M, Shimizu Y, Takahashi T, et al. Detection of human papillomavirus infection in oral squamous cell carcinoma: a cohort study of Japanese patients. J Oral Pathol Med. 2016;45(8):565–72.
Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P, et al. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42(10):987–93.
O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–85.
Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;6:401–10.
Cao R, Björndahl MA, Religa P, Clasper S, Garvin S, Galter D, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;4:333–45.
Morisada T, Oike Y, Yamada Y, Urano T, Akao M, Kubota Y, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105(12):4649–56.
Kubo H, Cao R, Bräkenhielm E, Mäkinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA. 2002;99(13):8868–73.
Saito Y, Nakagami H, Morishita R, Takami Y, Kikuchi Y, Hayashi H, et al. Transfection of human hepatocyte growth factor gene ameliorates secondary lymphedema via promotion of lymphangiogenesis. Circulation. 2006;114(11):1177–84.
Kreppel M, Scheer M, Drebber U, Ritter L, Zöller JE. Impact of podoplanin expression in oral squamous cell carcinoma: clinical and histopathologic correlations. Virchows Arch. 2010;456(5):473–82.
Bunget A, Fronie A, Afrem E, Corlan Puşcu D, Manolea H, Dan AR, et al. Microscopic aspects of angiogenesis and lymphangiogenesis in oral squamous cell carcinoma. Rom J Morphol Embryol. 2013;54(3):623–7.
Shao Z, Zhang WF, Chen XM, Shang ZJ. Expression of EphA2 and VEGF in squamous cell carcinoma of the tongue: correlation with the angiogenesis and clinical outcome. Oral Oncol. 2008;44(12):1110–7.
Naruse T, Yamamoto S, Yamada S, Takahashi H, Matsushita Y, Imayama N, et al. Immunohistochemical study of vascular endothelial growth factor-C/vascular endothelial growth factor receptor-3 expression in oral tongue squamous cell carcinoma: correlation with the induction of lymphangiogenesis. Oncol Lett. 2015;4:2027–34.
Holder N, Klein R. Eph receptors and ephrins: effectors of morphogenesis. Development. 1999;126(10):2033–44.
Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–75.
Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000;19(52):6043–52.
Kataoka H, Igarashi H, Kanamori M, Ihara M, Wang JD, Wang YJ, et al. Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer. Cancer Sci. 2004;95(2):136–41.
Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by EphrinB2 and its receptor EphB4. Cell. 1998;93(5):741–53.
Sharma GK, Dhillon VK, Masood R, Maceri DR. Overexpression of EphB4, EphrinB2, and epidermal growth factor receptor in papillary thyroid carcinoma: a pilot study. Head Neck. 2015;37(7):964–9.
Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T. Coexpression of EphB4 and ephrinB2 in tumor advancement of uterine cervical cancers. Gynecol Oncol. 2009;114(1):84–8.
Ozgür E, Heidenreich A, Dagtekin O, Engelmann U, Bloch W. Distribution of EphB4 and EphrinB2 in normal and malignant urogenital tissue. Urol Oncol. 2011;29(1):78–84.
Sobin LH, Gospodarowicz MK, Wittekind CH, editors. UICC TNM classification of malignant tumours. Hoboken: Wiley-Blackwell; 2009. p. 22–9.
Yamamoto E, Miyakawa A, Kohama G. Mode of invasion and lymph node metastasis in squamous cell carcinoma of the oral cavity. Head Neck Surg. 1984;5:938–47.
Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324(1):1–8.
Khan Z, Tiwari RP, Mulherkar R, Sah NK, Prasad GB, Shrivastava BR, et al. Detection of survivin and p53 in human oral cancer: correlation with clinicopathologic findings. Head Neck. 2009;8:1039–48.
Miyahara M, Tanuma J, Sugihara K, Semba I. Tumor lymphangiogenesis correlates with lymph node metastasis and clinicopathologic parameters in oral squamous cell carcinoma. Cancer. 2007;110(6):1287–94.
Watanabe S, Kato M, Kotani I, Ryoke K, Hayashi K. Lymphatic vessel density and vascular endothelial growth factor expression in squamous cell carcinomas of lip and oral cavity: a clinicopathological analysis with immunohistochemistry using antibodies to D2-40 VEGF-C and VEGF-D. Yonago Acta Med. 2013;56:29–37.
Agarwal D, Pardhe N, Bajpai M, Gupta S, Mathur N, Vanaki SS, et al. Characterization, localization and patterning of lymphatics and blood vessels in oral squamous cell carcinoma: a comparative study using D2-40 and CD-34 IHC marker. J Clin Diagn Res. 2014;10:86–9.
Nakaya H, Kawashiri S, Tanaka A, Noguchi N, Kato K, Hase T, et al. Influences of angiogenesis and lymphangiogenesis on cancerous invasion in experimentally induced tongue carcinoma. J Oral Pathol Med. 2005;34(2):87–92.
Goes RS, Carvalho JP, Almeida BG, Bacchi CE, Goes JC, Calil MA, et al. Intratumoral lymphatic vessel density in vulvar squamous cell carcinomas: a possible association with favorable prognosis. Int J Gynecol Pathol. 2012;1:8–14.
Acknowledgements
This study was approved by the appropriate ethical review boards, and participants provided written informed consent prior to participating in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saito, H., Oikawa, M., Kouketsu, A. et al. Immunohistochemical assessment of Eph/ephrin expression in oral squamous cell carcinoma and precursor lesions. Odontology 108, 166–173 (2020). https://doi.org/10.1007/s10266-019-00466-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-019-00466-y