Abstract
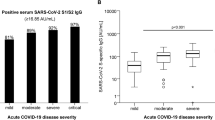
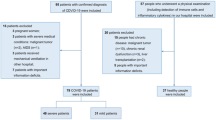
Medical follow-up of symptomatic patients after acute Coronavirus Disease 2019 (COVID-19) results in major burdens on patients and healthcare systems. The value of serological markers as part of this follow-up remains undetermined. We aimed to evaluate the clinical implications of serological markers for follow-up of acute COVID-19. For this purpose, we conducted an observational cohort study of patients 3 months after acute COVID-19. Participants visited a respiratory-clinic between October 2020 and March 2021, and completed pulmonary function tests (PFTs), serological tests, symptom-related questionnaires, and chest CT scans. Overall, 275 patients were included at a median of 82 days (IQR 64–111) post infection. 162 (59%) patients had diffusing capacity for carbon monoxide corrected for hemoglobin (DLCOc) below 80%, and 69 (25%) had bilateral chest abnormalities on CT scan. In multivariate analysis, anti-S levels were an independent predictor for DLCOc (β = − 0.14, p = 0.036). Anti-S levels were also associated with severe COVID-19 and older age, and correlated with anti-nucleocapsid (r = 0.30, p < 0.001) and antibodies to receptor binding domain (RBD, r = 0.37, p < 0.001). Other serological variables were not associated with clinical outcomes. In conclusion, symptomatic patients 3-months after COVID-19 had high respiratory symptomatic burden, in which anti-S levels were significantly associated with previous severe COVID-19 and DLCOc.



Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article. Due to ethical and privacy concerns the primary dataset cannot be made openly available. The study was done retrospectively and according to the regulations of our institution review board such data could be openly shared. Request for the dataset supporting our results can be made and will be given by the first author after approval.
References
Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226(9):1593–607.
O’Mahoney LL, Routen A, Gillies C, et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine. 2023;55: 101762.
Raghu G, Wilson KC. COVID-19 interstitial pneumonia: monitoring the clinical course in survivors. Lancet Respir Med. 2020;8(9):839–42.
Cutler DM. The Costs of Long COVID. JAMA Heal Forum. 2022;3(5):e221809–e221809. https://doi.org/10.1001/jamahealthforum.2022.1809.
Cutler DM, Summers LH. The COVID-19 pandemic and the $16 trillion virus. JAMA. 2020;324(15):1495–6. https://doi.org/10.1001/jama.2020.19759.
Freund O, Azolai L, Sror N, et al. Diagnostic delays among COVID-19 patients with a second concurrent diagnosis. J Hosp Med. 2023;18(4):321–8. https://doi.org/10.1002/jhm.13063.
Anastasio F, Barbuto S, Scarnecchia E, et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J. 2021;58(3).
Peluso MJ, Deitchman AN, Torres L, et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep. 2021;36(6): 109518.
Chan A, Martinez-Cajas J, Yip PM, et al. Evaluation and comparison of four quantitative SARS-CoV-2 serological assays in COVID-19 patients and immunized healthy individuals, cancer patients, and patients with immunosuppressive therapy. Clin Biochem. 2023;116:1–6.
Fogh K, Larsen TG, Hansen CB, et al. Self-reported long COVID and its association with the presence of SARS-CoV-2 antibodies in a danish cohort up to 12 months after infection. Microbiol Spectr. 2022;10(6): e0253722.
García-Abellán J, Padilla S, Fernández-González M, et al. Antibody response to SARS-CoV-2 is associated with long-term clinical outcome in patients with COVID-19: a longitudinal study. J Clin Immunol. 2021;41(7):1490–501. https://doi.org/10.1007/s10875-021-01083-7.
Centers for Disease Control and Prevention (CDC). Antibody testing interim guidelines for COVID-19 antibody testing in clinical and public health settings 2022 [cited 2023 Mar 4]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing/antibody-tests-guidelines.html.
Zhao Y-M, Shang Y-M, Song W-B, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25: 100463.
Drake TM, Riad AM, Fairfield CJ, et al. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, multicentre cohort study. Lancet. 2021;398(10296):223–37.
National Institutes of Health. COVID-19 treatment guidelines [cited 2021 Jan 12]. https://www.covid19treatmentguidelines.nih.gov/.
Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–32.
Augustin M, Schommers P, Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122.
Guler SA, Wohlfarth E, Berezowska S, Geiser TK, Ebner L, Funke-Chambour M. Performance of a diagnostic algorithm for fibrotic hypersensitivity pneumonitis. A case–control study. Respir Res. 2021;22(1):1–7. https://doi.org/10.1186/s12931-021-01727-7.
Yang X, Li Z, Wang B, et al. Prognosis and antibody profiles in survivors of critical illness from COVID-19: a prospective multicentre cohort study. Br J Anaesth. 2022;128(3):491–500.
Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–46. https://doi.org/10.1038/s41579-022-00846-2.
Faverio P, Luppi F, Rebora P, et al. Six-month pulmonary impairment after severe COVID-19: a prospective, multicentre follow-up study. Respiration. 2021;100(11):1078–87.
Legros V, Denolly S, Vogrig M, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18(2):318–27. https://doi.org/10.1038/s41423-020-00588-2.
Muecksch F, Wise H, Batchelor B, et al. Longitudinal serological analysis and neutralizing antibody levels in coronavirus disease 2019 convalescent patients. J Infect Dis. 2021;223(3):389–98.
Freund O, Weiss TE, Tau L, et al. Safety and outcomes of an early discharge strategy with oxygen home therapy in stable severe COVID-19 patients. Infect Dis (Lond, Engl). 2023;55(4):292–8.
Freund O, Tau L, Weiss TE, et al. Associations of vaccine status with characteristics and outcomes of hospitalized severe COVID-19 patients in the booster era. PLoS ONE. 2022;17(5):e0268050. https://doi.org/10.1371/journal.pone.0268050.
Bichara CDA, da Silva Graça Amoras E, Vaz GL, et al. Dynamics of anti-SARS-CoV-2 IgG antibodies post-COVID-19 in a Brazilian Amazon population. BMC Infect Dis. 2021;21(1):443. https://doi.org/10.1186/s12879-021-06156-x.
Lerum TV, Maltzahn NN, Aukrust P, et al. Persistent pulmonary pathology after COVID-19 is associated with high viral load, weak antibody response, and high levels of matrix metalloproteinase-9. Sci Rep. 2021;11(1):23205. https://doi.org/10.1038/s41598-021-02547-x.
Jia X, Cao S, Lee AS, et al. Anti-nucleocapsid antibody levels and pulmonary comorbid conditions are linked to post-COVID-19 syndrome. JCI Insight. 2022;7(13).
Guler SA, Ebner L, Aubry-Beigelman C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021;57(4).
Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1):163.
Wu X, Liu X, Zhou Y, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9(7):747–54.
Acknowledgements
None.
Funding
The authors received no funding for this work.
Author information
Authors and Affiliations
Contributions
ABS conceived and designed the study. OF drafted the manuscript. ABS supervised the study. OF, SF, SFR, NB, and EK performed data analysis. OW, NZ, SCR, RGV, AU, and AB performed data acquisition. All authors critically revised the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any relevant conflict of interests.
Ethical approval
The study was approved by the Barzilai Medical Center review board. All included patients singed informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Freund, O., Breslavsky, A., Fried, S. et al. Interactions and clinical implications of serological and respiratory variables 3 months after acute COVID-19. Clin Exp Med 23, 3729–3736 (2023). https://doi.org/10.1007/s10238-023-01139-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01139-5