Abstract
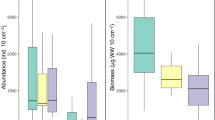
Free-living nematode distribution, abundance, diversity, and biological traits at Lake Bacalar were evaluated to assess the environmental quality of the water body. Using an Ekman grab, triplicate sediment samples were collected at 15 sampling sites. Nematodes were identified at family and genus levels, while abundance and diversity indices were calculated. Additionally, functional traits were calculated, and a PCA analysis was applied. The nematode fauna was represented by 6 orders, 18 families and 29 genera. Chromadorida had the highest number of taxa (9 genera), followed by Monhysterida (7 genera) and Enoplida (7 genera). Genera richness was higher at coarse sediments, with Simpson index values ranging 3.96−4.60, which were consistent with the Shannon index (H′ > 3 bits. ind−1). The maturity index varied from 2.5 to 3.1, with a higher percentage of cp-3–5 nematodes (> 55%). Multivariate analysis showed three nematode groups, one associated with chlorophyll a, pH, salinity, and silicates, the second group with dissolved oxygen, and organic matter, and a third group related with nutrient content in water. Biological traits showed a dominance of deposit feeders (37%) and epistrate feeders (35%). Nematode body shape was represented by slender (39%), medium plump (31%), while conical tails were dominant (68%) and the body length varied from < 500 to 3000 µm, with dominance of lengths > 500−750 (46%) and > 750−1250 (38%), organisms typical of oligotrophic lakes. Nematode assemblages, water chemical characteristics, mainly nutrient concentrations, and biological traits confirm the oligotrophic conditions at Lake Bacalar.



Similar content being viewed by others
References
Alves AS, Caetano A, Costa JL, Costa MJ, Marques JC (2015) Estuarine intertidal meiofauna and nematode communities as indicator of ecosystem’s recovery following mitigation measures. Ecol Indic 54:184–196
Andrassy I (1978) Nematoda. A checklist of the animals inhabiting European inland waters with accounts of their distribution and ecology. In: Illies J, Andrassy I (eds) Limnofauna Europacea. Gustav Fisher Verlag, Stuttgart, pp 98–117
Bazzanti M (2000) Macrobenthic nematodes as biological indicators in a Mediterranean low land river in Central Italy: a case study. Arch Hydrobiol 148:59–70
Beier S, Traunspurger W (2001) The meiofauna community of two small German streams as indicators of pollution. J Aquat Ecosyst Stress Recovery 8:387–405
Beier S, Traunspurger W (2003) Temporal dynamics of meiofauna communities in two small submountain carbonate streams with different grain size. Hydrobiologia 498:107–131
Bongers T (1990) The maturity index: an ecological measure of an environmental disturbance based on nematode species composition. Oecologia 83:14–19
Bongers T, Alkemade R, Yeates GW (1991) Interpretation of disturbance induced maturity decrease in marine nematode assemblages by means of the maturity index. Mar Ecol Prog Ser 76:135–142
Bremner J, Rogers SI, Frid CLJ (2003) Assessing functional diversity in marine benthic ecosystems: a comparison of approaches. Mar Ecol Prog Ser 254:11–25
Brinke M, Ristau K, Bergtold M, Höss S, Clauss E, Heininger P, Traunspurger W (2011) Using meiofauna to assess pollutants in freshwater sediments. A microcosm study with cadmium. Environ Tox Chem 30:427–438
Buchanan JB (1984) Sediment analysis. In: Holme A, McIntyre AD (eds) Methods for the study of marine benthos. Blackwell, London, pp 41–65
Chalcraft DR, Resetarits WJ (2003) Mapping functional similarity of predators on the basis of trait similarities. Amer Nat 162:390–402
Clarke KR, Gorley RN (2006) PRIMER v5. User Manual/tutorial, PRIMER-E, Plymouth, pp 91
Contreras-Espinosa F (1984) Manual de Técnicas Hidrobiológicas. Universidad Autónoma Metropolitana-Iztapalapa, México, p 141
De Jesús-Navarrete A (1993) Nematodos de la Laguna de Buenavista, Quintana Roo, México. Rev Biol Trop 41(3):649–652
De Jesús-Navarrete A, Yanez-Montalvo A, Falcon L, Vargas-Espositos A (2021) Nematode fauna associated with freshwater microbialites in Bacalar Lake, Quintana Roo, Mexico. Limnology 22:347–355
Dean WE Jr (1974) Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition: comparison with other methods. J Sediment Petrol 12:242–248
Decramer W, Coomans A (1994) A compendium of our knowledge of the free-living nematofauna of ancient lakes. Archiv Fu¨r Hydrobiologie Beiheft Ergebnisse Der Limnologie 44:173–181
Eyualem A, Mees J, Coomans A (2001) Nematode communities of Lake Tana and other inland water bodies of Ethiopia. Hydrobiologia 462:41–63
Eyualem A, Andrássy I, Traunspurger W (eds) (2006) Freshwater nematodes: ecology and taxonomy. CABI Publishing, Cambridge
Eyualem A, Decramer W, De Ley P (2008) Global diversity of nematodes (Nematoda) in fresh water. Hydrobiologia 595:67–78
Ferris H, Bongers T (2009) Indices developed specifically to analyze of nematode assemblages. In: Wilson MJ, Kakouli-Duarte T (eds). Nematodes as indicators. CAB, International, pp 124–145
Flach ZSP, Ozorio CP, Melo AS (2012) Alpha and beta components of diversity of freshwater nematodes at different spatial scales in subtropical coastal lakes. Fundam Appl Limnol 180:249–258
Fleeger JW, Carman KR, Weisenhorn PB, Sofranko H, Marshall T, Thistle D, Barry JP (2006) Simulated sequestration of anthropogenic carbon dioxide at a deep-sea site: effects on nematode abundance and biovolume. Deep Sea Res Part I: Oceanogr Res Papers 53:1135–1147
Fleeger JW, Johnson DS, Carman KR, Weisenhorn PB, Gabriele A, Thistle D, Barry JP (2010) The response of nematodes to deep-sea CO 2 sequestration: a quantile regression approach. Deep Sea Res Part I: Oceanogr Res Papers 57:696–707
Fraschetti S, Gambi C, Giangrande A, Musco L, Terlizzi A, Danovaro R (2006) Structural and functional response of meiofauna rocky assemblages to sewage pollution. Mar Pollut Bull 52:540–548
Gamboa-Pérez HC, Schmitter-Soto JJ (1999) Distribution of ciclid fishes in the littoral of lake Bacalar, Yucatan Peninsula. Environ Biol Fishes 54:35–43
Gischler E, Gibson MA, Oschmann W (2008) Giant Holocene freshwater microbialites, Laguna Bacalar, Quintana Roo, Mexico. Sedimentology 55:1293–1309
Heininger P, Höss S, Claus E, Jurgen P, Traunspurger W (2007) Nematode communities in contaminated river sediments. Environ Pollut 146:64–76
Hering D, Moog O, Sandelin L, Verdonschot PFM (2004) Overview and application of the AQEM assessment system. Hydrobiologia 516:1–21
Hernández Arana H, Vega-Zepeda A, Ruiz-Zarate MA, Falcon LI, López-Adame H, Herrera Silveira J, Kaster J (2015) Transverse Coastal Corridor: From Freshwater Lakes to Coral Reefs Ecosystems. In: Islebe GA, Calmé S, León-Cortes J, Schmook B (eds) Biodiversity and Conservation of the Yucatán Peninsula. Springer, London, pp 355–376
Heyns J (1976) Preliminary results of a survey of freshwater nematodes in South Africa. J Limnol Soc South Africa 2:43–45
Höss S, Claus E, Von der Ohe PC, Brinke M, Güde H, Heininger P, Traunspurger W (2011) Nematode species at risk e a metric to assess pollution in soft sediments of freshwaters. Environ Int 37:940–949
Isphording WC (1975) The physical geology of Yucatan. Trans Gulf Coast Assoc Geol Soc 25:231–262
Jacobs LJ (1984) The free-living inland aquatic nematodes of Africa—a review. Hydrobiologia 113:259–291
Jensen P (1987) Feeding ecology of free-living marine nematodes. Mar Ecol Prog Ser 35:187–196
Lambshead PJD (1986) Sub-catastrophic sewage and industrial waste contamination as revealed by marine nematode faunal analysis. Mar Ecol Prog Ser 29:247–260
Lara ME (1993) Divergent Wrench Faulting in the Belize Southern Lagoon - Implications for Tertiary Caribbean Plate Movements and Quaternary Reef Distribution. Aapg Bull Amer Assoc Petro Geol 77:1041–1063
Martínez-Lladó X, Gibert O, Martí V, Díez S, Romo J, Bayona JM, de Pablo J (2007) Distribution of polycyclic aromatic hydrocarbons (PAHs) and tributyltin (TBT) in Barcelona harbor sediments and their impact on benthic communities. Environ Pollut 149:104–113
Moreno M, Vezzulli L, Marin V, Laconi P, Albertelli G, Fabiano M (2008) The use of meiofauna diversity as an indicator of pollution in harbours. ICES J Mar Sci 65:1428–1435
Moreno M, Semprucci F, Vezzulli L, Balsamo M, Fabiano M, Albertelli G (2011) The use of nematodes in assessing ecological quality status in the Mediterranean coastal ecosystems. Ecol Indic 11:328–336
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chem Acta 27:31–36
Ocaña A, Picazo JS (1991) Study on nematode species encountered in the Monachil River (Granada, Spain): response to organic pollution. Erhandlungen Der Internationalen Vereinigung Der Theoretischen Und Angewandten Limnologie 24:2729–2737
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford
Pawhestri SW, Hidayat JW, Putro SP (2015) Assessment of Water Quality Using Macrobenthos as Bioindicator and Its Application on Abundance-Biomass Comparison (ABC) Curves. Int J Sci Eng 8(2):84–87. https://doi.org/10.12777/ijse.8.2.84-87
Pérez L, Bugja R, Lorenschat L, Brenner M, Curtis J, Hoetzmann P, Islebe G, Scharf B, Schwalb A (2011) Aquatic ecosystems of the Yucatán Peninsula (Mexico), Belize and Guatemala. Hydrobiologia 661:407–433
Platt H, Shaw MKM, Lambshead PJD (1984) Nematode species abundance patterns and their use in the detection of environmental perturbations. Hydrobiologia 118:56–66
Ptatscheck C, Traunspurger W (2020) The ability to get everywhere: dispersal modes of free-living, aquatic nematodes. Hydrobiologia 847:3519–3547. https://doi.org/10.1007/s10750-020-04373-0
Ristau K, Spann N, Traunspurger W (2015) Species and trait compositions of freshwater nematodes as indicative descriptors of lake eutrophication. Ecol Indic 53:196–205
Schmitter-Soto JJ, Comin FA, Escobar-Briones E, Herrera-Silveira J, Alcocer JR, Suárez-Morales E, Elías-Gutierrez M, Díaz-Arce V, Marín LE, Steinich B (2002) Hydrogeochemical and biological characteristics of cenotes in the Yucatan Peninsula (SE Mexico). Hydrobiologia 467:215–228
Schratzberger M, Warr KJ, Rogers SI (2007) Functional diversity of nematode communities in the southwestern North Sea. Mar Environ Res 63:368–389
Smith DG (2001) Pennak´s freshwater invertebrates of the United States: porifera to crustacea. Wiley, New York
Soetaert K, Muthumbi A, Heip C (2002) Size and shape of ocean margin nematodes: morphological diversity and depth-related patterns. Mar Ecol Prog Ser 242:179–193
Soetaert K, Franco M, Lampadariou N, Muthumbi A, Steyaert M, Vandepitte L, Vanaverbeke J (2009) Factors affecting nematode biomass, length, and width from the shelf to the deep sea. Mar Ecol Prog Ser 392:123–132
Thistle D, Sherman KM (1985) The nematode fauna of a deep-sea site exposed to strong near-bottom currents. Deep Sea Res Oceanogr Res Papers 32:1077–1088
Thistle D, Lambshead PJD, Sherman KM (1995) Nematode tail-shape groups respond to environmental differences in the deep sea. Vie Milieu 45:107–115
Thorp JH, Covich AP (eds) (1991) Ecology and classification of North American Freshwater invertebrates. Academic Press, San Diego
Traunspurger W (1997) Bathymetric, seasonal and vertical distribution of feeding-types of nematodes in an oligotrophic lake. Vie Milieu 47:1–7
Traunspurger W (2002) Nematoda. In: Rundle SD, Robertson AL, Schmid-Araya JM (eds) Freshwater meiofauna: biology and ecology. Backhuys Publishers, Leiden, pp 63–104
Traunspurger W, Höss S, Witthöft-Mühlmann A, Wessels M, Güde H (2012) Meiobenthic community patterns of oligotrophic and deep Lake Constance in relation to water depth and nutrients. Fundam Appl Limnol 180:233–248. https://doi.org/10.1127/1863-9135/2012/0144
Traunspurger W, Witthöft-Mühlmann A, Höss S (2020) Free-living nematode communities in a large and deep oligotrophic lake in Europe: comparison of different depth zones of Lake Constance (Germany). Nematology 1:1–19
U.S. EPA (2001) National Coastal Assessment: Field Operations Manual. U.S. Environmental Protection Agency, Office of Research and Development, National Health and Environmental Effects Research Laboratory, Gulf Ecology Division, Gulf Breeze, F.L. EPA 620/R-01/003. pp 72
Vanaverbeke J, Steyaert M, Soetaert K, Rousseau V, Van Gansbeke D, Parent JY, Vincx M (2004) Changes in structural and functional diversity of nematode communities during a spring phytoplankton bloom in the southern North Sea. J Sea Res 52:281–292
Vanaverbeke J, Deprez T, Vincx M (2007) Changes in nematode communities at the long-term sand extraction of the Kwinte-bank (Southern Bight of the North Sea). Mar Pollut Bull 54:1351–1360
Venturini N, Muniz P, Rodríguez M (2004) Macrobenthic subtidal communities in relation to sediment pollution: the phylum-level meta-analysis approach in a south-eastern coastal region of South America. Mar Biol 144:119–126
Vollenweider RA, Giovanardi F, Montanari G, Rinaldi A (1998) Characterization of the trophic conditions of marine coastal waters with special reference to the NW Adriatic Sea: Proposal for a trophic scale, turbidity and generalized water quality index. Environmetrics 9:329–357
Warwick RM (1981) The nematode /copepod ratio and it use in pollution ecology. Mar Pollut Bull 12:329–333
Wu HC, Chen PC, Tsay TT (2010) Assessment of nematode community structure as a bioindicator in river monitoring. Environ Pollut 158:1741–1747
Yanez-Montalvo A, Gómez-Acata S, Águila B, Hernández-Arana H, Falcón LI et al (2020) The microbiome of modern microbialites in Bacalar Lagoon, Mexico. PLoS ONE 15(3):e0230071
Zullini A (1974) The nematological population of the Po River. Boll Zool 41:183–210
Zullini A (1976) Nematodes as indicators of river pollution. Nematol Mediterr 4:13–22
Zullini A (2010) Identification manual for freshwater nematode genera. Universidade di Milano-Bicocca, Milano, p 112
Acknowledgements
This work was part of the project “Monitoring of water quality from Lakes of Quintana Roo” financed by FOMIX-QROO-2009-C01-123254. The critical comments of Ashleigh Smythe and two anonymous reviewers improved the manuscript substantially.
Funding
This work was funded by Consejo Nacional de Ciencia y Tecnología (Grant no. COI-123254).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Koji Tojo.
Rights and permissions
About this article
Cite this article
de Jesús-Navarrete, A., Legorreta, T.Á. Biological traits analysis of free-living nematodes as indicators of environmental quality at Lake Bacalar, Mexico. Limnology 23, 355–364 (2022). https://doi.org/10.1007/s10201-021-00693-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-021-00693-9