Abstract
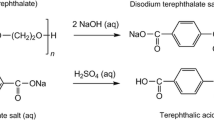
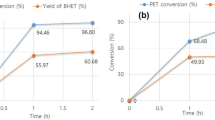
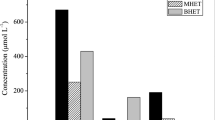
Polyvinyl chloride (PVC)-coated poly(ethylene terephthalate) (PET) woven fibers are one of the hardest-to-recycle polymeric materials. Herein we investigate the possibility of recycling both PVC and PET through alkaline hydrolysis of PET. The coated woven fabrics were treated with NaOH, hydrolyzing the PET fibers into water-soluble sodium terephthalate, while the PVC could be removed by filtration. The PET fibers were completely hydrolyzed between 120 and 180 °C in the presence of 1 M NaOH solution, quantitatively yielding terephthalic acid. A minimum PVC dechlorination rate of 1% was simultaneously achieved at 120 °C. Furthermore, no alkaline hydrolysis of the plasticizer contained in the PVC, di(2-ethylhexyl)phthalate, was observed. Thus, the possibility of simultaneously recycling PET and PVC from PVC-coated woven fabrics was demonstrated. Kinetic analyses of PET hydrolysis and PVC dechlorination revealed that the simultaneously occurring reaction processes did not affect the progress of each other. Thus, the absence of interactions between PET, PVC, or their degradation products enables the design of a simplified recycling process without considering the interactions between the materials derived from coated woven fabrics.







Similar content being viewed by others
References
Toyoda H (2012) Recyclable coated fabric using kenaf fiber for architectural membrane structure applications. Fabr Archit 24:7–16
The Ministry of Land I, Transport and Tourism Order for Enforcement of the Building Standards Act, the provision of Article 39. http://law.e-gov.go.jp/htmldata/S25/S25SE338.html. Accessed 19 Apr 2017
Van Krevelen DW (1997) Part VII—introduction to comprehensive tables. In: Properties of polymers (Third, completely revised edition), pp 759–823
Fukushima M, Shioya M, Wakai K, Ibe H (2009) Toward maximizing the recycling rate in a sapporo waste plastics liquefaction plant. J Mater Cycles Waste Manage 11:11–18
Fukushima M, Wu B, Ibe H, Wakai K, Sugiyama E, Abe H, Kitagawa K, Tsuruga S, Shimura K, Ono E (2010) Study on dechlorination technology for municipal waste plastics containing polyvinyl chloride and polyethylene terephthalate. J Mater Cycles Waste Manage 12:108–122
Bhaskar T, Kaneko J, Muto A, Sakata Y, Jakab E, Matsui T, Uddin MA (2004) Pyrolysis studies of PP/PE/PS/PVC/HIPS-Br plastics mixed with PET and dehalogenation (Br, Cl) of the liquid products. J Anal Appl Pyrolysis 72:27–33
Bhaskar T, Uddin MA, Kaneko J, Kusaba T, Matsui T, Muto A, Sakata Y, Murata K (2003) Liquefaction of mixed plastics containing PVC and dechlorination by calcium-based sorbent. Energy Fuels 17:75–80
Born JGP, Mulder P, Louw R (1993) Fly ash mediated reactions of phenol and monochlorophenols: oxychlorination, deep oxidation, and condensation. Environ Sci Technol 27:1849–1863
The European Council of Vinyl Manufacturers PVC recycling by application. http://www.pvc.org/en/p/pvc-recycling-by-application. Accessed 13 July 2016
Grause G, Hirahashi S, Toyoda H, Kameda T, Yoshioka T (2015) Solubility parameters for determining optimal solvents for separating PVC from PVC-coated PET fibers. J Mater Cycles Waste Manag 19:612–622
Ravens DAS, Ward IM (1961) Chemical reactivity of polyethylene terephthalate. Hydrolysis and esterification reactions in the solid phase. Trans Faraday Soc 57:150
Zimmerman H, Kim NT (1980) Investigations on thermal and hydrolytic degradation of poly(ethylene terephthalate). Polym Eng Sci 20:680–683
Campanelli JR, Cooper DG, Kamal MR (1993) A kinetic study of the hydrolytic degradation of polyethylene terephthalate at high temperatures. J Appl Polym Sci 48:443–451
Zope VS, Mishra S (2008) Kinetics of neutral hydrolytic depolymerization of PET (polyethylene terephthalate) waste at higher temperature and autogenious pressures. J Appl Polym Sci 110:2179–2183
Ramsden MJ, Phillips JA (1996) Factors influencing the kinetics of the alkaline depolymerisation of poly(ethylene terephthalate). I: the effect of solvent. J Chem Technol Biotechnol 67:131–136
Wan B-Z, Kao C-Y, Cheng W-H (2001) Kinetics of depolymerization of poly(ethylene terephthalate) in a potassium hydroxide solution. Ind Eng Chem Res 40:509–514
Karayannidis GP, Chatziavgoustis AP, Achilias DS (2002) Poly(ethylene terephthalate) recycling and recovery of pure terephthalic acid by alkaline hydrolysis. Adv Polym Technol 21:250–259
Kumar S, Guria C (2005) Alkaline hydrolysis of waste poly(ethylene terephthalate): a modified shrinking core model. J Macromol Sci Part A 42:237–251
Yoshioka T, Okayama N, Okuwaki A (1998) Kinetics of hydrolysis of PET powder in nitric acid by a modified shrinking-core model. Ind Eng Chem Res 37:336–340
Yoshioka T, Motoki T, Okuwaki A (2001) Kinetics of hydrolysis of poly(ethylene terephthalate) powder in sulfuric acid by a modified shrinking-core model. Ind Eng Chem Res 40:75–79
Achilias DS, Karayannidis GP (2004) The chemical recycling of PET in the framework of sustainable development. Water Air Soil Pollut Focus 4:385–396
Shin S-M, Watanabe S, Yoshioka T, Okuwaki A (1997) Dehydrochlorination behavior of agricultural PVC polymer films in alkaline solution at elevated temperatures. Nippon Kagaku Kaishi 1:64–68
Shin SM, Jeon HS, Kim YH, Yoshioka T, Okuwaki A (2002) Plasticizer leaching from flexible PVC in low temperature caustic solution. Polym Degrad Stab 78:511–517
Kumagai S, Grause G, Kameda T, Yoshioka T (2014) Simultaneous recovery of benzene-rich oil and metals by steam pyrolysis of metal-poly(ethylene terephthalate) composite waste. Environ Sci Technol 48:3430–3437
Kumagai S, Hosaka T, Kameda T, Yoshioka T (2016) Pyrolysis and hydrolysis behaviors during steam pyrolysis of polyimide. J Anal Appl Pyrolysis 120:75–81
Kumagai S, Morohoshi Y, Grause G, Kameda T, Yoshioka T (2015) Pyrolysis versus hydrolysis behavior during steam decomposition of polyesters using 18O-labeled steam. RSC Adv 5:61828–61837
Hashimoto K, Suga S, Wakayama Y, Funazukuri T (2007) Hydrothermal dechlorination of PVC in the presence of ammonia. J Mater Sci 43:2457–2462
Siddiqui MN, Achilias DS, Redhwi HH, Bikiaris DN, Katsogiannis K-AG, Karayannidis GP (2010) Hydrolytic depolymerization of PET in a microwave reactor. Macromol Mater Eng 295:575–584
López-Fonseca R, González-Marcos MP, González-Velasco JR, Gutiérrez-Ortiz JI (2009) A kinetic study of the depolymerisation of poly(ethylene terephthalate) by phase transfer catalysed alkaline hydrolysis. J Chem Technol Biotechnol 84:92–99
Ikenaga K (2008) Microwave-titanium oxide catalyzed chemical depolymerization of waste PET. Kagaku Kogyo 59:494–499
Roh H-D, Bae D (2000) Process for manufacturing terephthalic acid. US Patent (US 6075163 A)
Jr. GEB, O’Brien RC (1976) Method for recovering terephthalic acid ethylene glycol from polyester materials. US Patent (US 3952053 A)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumagai, S., Hirahashi, S., Grause, G. et al. Alkaline hydrolysis of PVC-coated PET fibers for simultaneous recycling of PET and PVC. J Mater Cycles Waste Manag 20, 439–449 (2018). https://doi.org/10.1007/s10163-017-0614-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-017-0614-4