Abstract
Background
There are two approaches for treating cytomegalovirus (CMV) infection occurring after kidney transplantation (KTx). One is preemptive therapy in which treatment is started after confirming positive CMV antigenemia using periodic antigenemia assay. The other approach is prophylactic therapy in which oral valganciclovir (VGCV) is started within 10 days after KTx and continued for 200 days. The Transplantation Society guidelines recommend prophylactic therapy for high-risk (donor’s CMV-IgG antibody positive and recipient’s negative) pediatric recipients. However, the adequate dose and side effects of VGCV are not clear in children, and there is no sufficient information about prophylaxis for Japanese pediatric recipients.
Methods
A single-center retrospective analysis was conducted on case series of high-risk pediatric patients who underwent KTx and received oral VGCV prophylaxis at the Department of Pediatric Nephrology, Tokyo Women’s Medical University, between August 2018 and March 2019. Data were collected using medical records.
Results
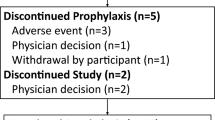
The dose of administration was 450 mg in all the study patients (n = 5). Reduction or discontinuation was required in four of five patients due to adverse events, which included neutropenia in one patient, anemia in two patients, and neutropenia and digestive symptoms in one patient. Late-onset CMV disease occurred in all patients. No seroconversion was observed during prophylaxis.
Conclusions
Our preliminary study suggests that the dosage endorsed by The Transplantation Society may be an overdose for Japanese pediatric recipients. Further studies are required to examine the safety and efficacy of VGCV prophylaxis in Japanese pediatric recipients.
Similar content being viewed by others
References
Rubin RH. The pathogenesis and clinical management of cytomegalovirus infection in the organ transplant recipient: the end of the ‘silo hypothesis.’ Curr Opin Infect Dis. 2007;20:399–407.
Sagedal S, Hartmann A, Nordal KP, et al. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int. 2004;66:329–37.
Atabani SF, Smith C, Atkinson C, et al. Cytomegalovirus replication kinetics in solid organ transplant recipients managed by preemptive therapy. Am J Transplant. 2012;12:2457–64.
Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2018 annual data report: kidney. Am J Transplant. 2020;20(Suppl s1):20–130.
Kotton CN, Kumar D, Caliendo AM, et al. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018;102:900–31.
Tan BH. CMV prophylaxis—to do or not to do, that is the question. Nephrol Dial Transplant. 2006;21:1757–61.
Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37.
Uemura O, Nagai T, Ishikura K, et al. Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol. 2014;18:626–33.
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098.
Hattori M, Mieno M, Shishido S, et al. Outcomes of pediatric ABO-incompatible living kidney transplantations from 2002 to 2015: an analysis of the Japanese kidney transplant registry. Transplantation. 2018;102:1934–42.
Varela-Fascinetto G, Benchimol C, Reyes-Acevedo R, et al. Tolerability of up to 200 days of prophylaxis with valganciclovir oral solution and/or film-coated tablets in pediatric kidney transplant recipients at risk of cytomegalovirus disease. Pediatr Transplant. 2017. https://doi.org/10.1111/petr.12833.
Höcker B, Zencke S, Krupka K, et al. Cytomegalovirus infection in pediatric renal transplantation and the impact of chemoprophylaxis with (Val-)Ganciclovir. Transplantation. 2016;100:862–70.
Gabardi S, Asipenko N, Fleming J, et al. Evaluation of low- versus high-dose valganciclovir for prevention of cytomegalovirus disease in high-risk renal transplant recipients. Transplantation. 2015;99:1499–505.
Stevens DR, Sawinski D, Blumberg E, Galanakis N, Bloom RD, Trofe-Clark J. Increased risk of breakthrough infection among cytomegalovirus donor-positive/recipient-negative kidney transplant recipients receiving lower-dose valganciclovir prophylaxis. Transpl Infect Dis. 2015;17:163–73.
Vaudry W, Ettenger R, Jara P, et al. Valganciclovir dosing according to body surface area and renal function in pediatric solid organ transplant recipients. Am J Transplant. 2009;9:636–43.
Åsberg A, Bjerre A, Neely M. New algorithm for valganciclovir dosing in pediatric solid organ transplant recipients. Pediatr Transplant. 2014;18:103–11.
Pappo A, Peled O, Berkovitch M, et al. Efficacy and safety of a weight-based dosing regimen of valganciclovir for cytomegalovirus prophylaxis in pediatric solid-organ transplant recipients. Transplantation. 2019;103:1730–5.
Uemura O, Honda M, Matsuyama T, Ishikura K, Hataya H, Nagai T, Ikezumi Y, Fujita N, Ito S, Iijima K, Japanese Society for Pediatric Nephrology, the Committee of Measures for Pediatric CKD. Is the new Schwartz equation derived from serum creatinine and body length suitable for evaluation of renal function in Japanese children? Eur J Pediatr. 2012;171:1401–4.
Halleck F, Khadzhynov D, Schrezenmeier E, Lehner L, Budde K, Staeck O. Prolonged low-dose prophylaxis with valganciclovir in cytomegalovirus-negative recipients of kidney transplants from cytomegalovirus-positive donors allows seroconversion and prevents cytomegalovirus disease. Transplant Proc. 2017;49:2280–4.
Nanmoku K, Shinzato T, Kubo T, Shimizu T, Kimura T, Yagisawa T. Prevention of late-onset cytomegalovirus infection and disease in donor-positive/recipient-negative kidney transplant recipients using low-dose valganciclovir. Transplant Proc. 2018;50:124–9.
Humar A, Mazzulli T, Moussa G, et al. Clinical utility of cytomegalovirus (CMV) serology testing in high-risk CMV D+/R− transplant recipients. Am J Transplant. 2005;5:1065–70.
Acknowledgments
We would like to express our sincere appreciation to all related parties and staff at the Department of Pediatric Nephrology of TWMU who cooperated in this study and MARUZEN-YUSHODO Co., Ltd. (https://kw.maruzen.co.jp/kousei-honyaku/) for the English language editing.
Author information
Authors and Affiliations
Contributions
TI designed the study and wrote the initial draft of the manuscript. KM and HB contributed to analysis and interpretation of data and assisted in the preparation of the manuscript. TA, YS, SI, AS, NK, TY, KI, MT, KS, and MH contributed to data collection and critically reviewed the manuscript. MH supervised the findings of this work. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This study was conducted in accordance with the ethical principles set out in the Declaration of Helsinki and the ethical guidelines for epidemiological studies issued by the Ministry of Health, Labour and Welfare, Japan. The Ethics Committee of the TWMU approved the study (approval no. 5528).
Informed consent
Informed consent was waived by giving the patients' parents an opportunity to opt out.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Iida, T., Miura, K., Ban, H. et al. Valganciclovir prophylaxis for cytomegalovirus infection in pediatric kidney transplant recipients: a single-center experience. Clin Exp Nephrol 25, 531–536 (2021). https://doi.org/10.1007/s10157-021-02020-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-021-02020-z