Abstract
Background
The aim of this study was to analyze the incidence, patterns and prognostic factors of recurrence in patients with complicated colon cancer who had emergency surgery within 24 h of admission.
Methods
A retrospective observational study was performed on patients with obstructing or perforated colon cancer having resection with curative intent between 1996 and 2014 at a single center. Data were obtained from a prospectively maintained database. Patients who had rectal cancer, iatrogenic endoscopic perforation, stage IV disease, palliative surgery, a colonic stent or decompressive colostomy were excluded.
Results
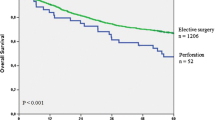
The study included 393 patients. Obstruction was observed in 320 patients (81.4%) and perforation in 73 (18.6%). Hartmann’s procedure was more frequently performed by general surgeons (7.5% vs 23.3%; p = 0.023). 30-day postoperative mortality was 13.5% (53/393), including 47 (14.7%) obstructed and 6 (8.2%) perforated patients. Postoperative complications (Clavien–Dindo III–IV) occurred in 87 patients (22.1%), including 68 (21.2%) of obstructed and 19 (26.0%) of perforated patients. Anastomotic dehiscence was diagnosed in 52 of 329 (15.8%) patients with primary anastomosis and was higher in the obstructing group than in the perforated group (17.4% vs 7.6%). There was a significantly higher anastomotic dehiscence rate after procedures performed by general surgeons when compared with those performed by colorectal surgeons (10.3% vs 21.3%; p = 0.005; OR 2.81, 95% CI 1.4–5.9). With a median follow-up of 6 years, the recurrence rate was 30.1% (67.4% distant, 22.8% local, 9.8% both). Overall and cancer-related survivals were 68.7% and 77.8%, respectively. The presence of positive nodes, male gender, anastomotic dehiscence and diffuse peritonitis were independent predictors for local recurrence while type of surgeon (general) was an independent factor for distant recurrence.
Conclusions
Male gender, diffuse peritonitis, positive lymph nodes, type of surgeon and postoperative anastomotic dehiscence significantly influence recurrence of colorectal cancer in this series.


Similar content being viewed by others
References
Chen TM, Huang YT, Wang GC (2017) Outcome of colon cancer initially presenting as colon perforation and obstruction. World J Surg Oncol 15:164
Biondo S, Kreisler E, Millan M et al (2008) Differences in patient postoperative and long-term outcomes between obstructive and perforated colonic cancer. Am J Surg 195:427–432
Frago R, Biondo S, Millan M et al (2011) Differences between proximal and distal obstructing colonic cancer after curative surgery. Colorectal Dis 13:e116–e122
Yang XF, Pan K (2014) Diagnosis and management of acute complications in patients with colon cancer: bleeding, obstruction, and perforation. Chin J Cancer Res 26:331–340
Ho YH, Siu SK, Buttner P, Stevenson A, Lumley J, Stitz R (2010) The effect of obstruction and perforation on colorectal cancer disease-free survival. World J Surg 34:1091–1101
McArdle CS, Hole DJ (2004) Influence of volume and specialization on survival following surgery for colorectal cancer. Br J Surg 91:610–617
Biondo S, Martí-Ragué J, Kreisler E et al (2005) A prospective study of outcomes of emergency and elective surgeries for complicated colonic cancer. Am J Surg 189:377–383
Baer C, Menon R, Bastawrous S, Bastawrous A (2017) Emergency presentations of colorectal cancer. Surg Clin North Am 97:529–545
Hogan J, Samaha G, Burke J et al (2015) Emergency presenting colon cancer is an independent predictor of adverse disease-free survival. Int Surg 100:77–86
Biondo S, Kreisler E, Millan M et al (2010) Impact of surgical specialization on emergency colorectal surgery outcomes. Arch Surg 145:79–86
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Biondo S, Kreisler E, Millan M et al (2007) Long-term results of emergency surgery for colon cancer compared with elective surgery. Cir Esp 82:89–98
Iversen LH, Bülow S, Christensen IJ, Laurberg S, Harling H, Danish Colorectal Cancer Group (2008) Postoperative medical complications are the main cause of early death after emergency surgery for colonic cancer. Br J Surg 95:1012–1019
Weixler B, Warschkow R, Ramser M et al (2016) Urgent surgery after emergency presentation for colorectal cancer has no impact on overall and disease-free survival: a propensity score analysis. BMC Cancer 16:208
Smith JA, King PM, Lane RH, Thompson MR (2003) Evidence of the effect of specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg 90:583–592
Degett TH, Dalton SO, Christensen J, Søgaard J, Iversen LH, Gögenur I (2019) Mortality after emergency treatment of colorectal cancer and associated risk factors-a nationwide cohort study. Int J Colorectal Dis 34(1):85–95
Cortet M, Grimault A, Cheynel N, Lepage C, Bouvier AM, Faivre J (2013) Patterns of recurrence of obstructing colon cancers after surgery for cure: a population-based study. Colorectal Dis 15:1100–1106
Asano H, Kojima K, Ogino N, Fukano H, Ohara Y, Shinozuka N (2017) Postoperative recurrence and risk factors of colorectal cancer perforation. Int J Colorectal Dis 32:419–424
Crozier JE, Leitch EF, McKee RF, Anderson JH, Horgan PG, McMillan DC (2009) Relationship between emergency presentation, systemic inflammatory response, and cancer-specific survival in patients undergoing potentially curative surgery for colon cancer. Am J Surg 197(4):544–549
Manfredi S, Bouvier AM, Lepage C, Hatem C, Dancourt V, Faivre J (2006) Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg 93:1115–1122
Read TE, Mutch MG, Chang BW et al (2002) Locoregional recurrence and survival after curative resection of adenocarcinoma of the colon. J Am Coll Surg 195:33–40
Harris GJ, Church JM, Senagore AJ et al (2002) Factors affecting local recurrence of colonic adenocarcinoma. Dis Colon Rectum 45:1029–1034
Chen HS, Sheen-Chen SM (2000) Obstruction and perforation in colorectal adenocarcinoma: an analysis of prognosis and current trends. Surgery 127:370–376
Sjövall A, Granath F, Cedermark B, Glimelius B, Holm T (2007) Loco-regional recurrence from colon cancer: a population-based study. Ann Surg Oncol 14:432–440
Sugawara K, Kawaguchi Y, Nomura Y, Koike D, Nagai M, Tanaka N (2017) Insufficient lymph node sampling in patients with colorectal cancer perforation is associated with an adverse oncological outcome. World J Surg 41:295–305
Runkel NS, Hinz U, Lehnert T, Buhr HJ, Herfarth Ch (1998) Improved outcome after emergency surgery for cancer of the large intestine. Br J Surg 85:1260–1265
Jessup JM, Stewart A, Greene FL, Minsky BD (2005) Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA 294:2703–2711
O’Connor ES, Greenblatt DY, Lo Conte NK et al (2011) Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 29:3381–3388
Quasar Collaborative Group, Gray R, Barnwell J, McConkey C et al (2007) Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 370:2020–2029
Barbas AS, Turley RS, Mantyh CR, Migaly J (2012) Effect of surgeon specialization on long-term survival following colon cancer resection at an NCI-designated cancer center. J Surg Oncol 106:219–223
Oliphant R, Nicholson GA, Horgan PG, Molloy RG, McMillan DC, Morrison DS, West of Scotland Colorectal Cancer Managed Clinical Network (2013) Contribution of surgical specialization to improved colorectal cancer survival. Br J Surg 100:1388–1395
Hall GM, Shanmugan S, Bleier JI, Jeganathan AN, Epstein AJ, Paulson EC (2016) Colorectal specialization and survival in colorectal cancer. Colorectal Dis 18:O51–O60
Alonso S, Pascual M, Salvans S, Mayol X, Mojal S, Gil MJ, Grande L, Pera M (2015) Postoperative intra-abdominal infection and colorectal cancer recurrence: a prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur J Surg Oncol 41(2):208–214
Sánchez-Velázquez P, Pera M, Jiménez-Toscano M, Mayol X, Rogés X, Lorente L, Iglesias M, Gallén M (2018) Postoperative intra-abdominal infection is an independent prognostic factor of disease-free survival and disease-specific survival in patients with stage II colon cancer. Clin Transl Oncol 20(10):1321–1328
Goto S, Hasegawa S, Hida K et al (2017) Study Group for Nomogram of the Japanese Society for Cancer of the Colon and Rectum. Multicenter analysis of impact of anastomotic leakage on long-term oncologic outcomes after curative resection of colon cancer. Surgery 162:317–324
Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H (2014) Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg 259:930–938
Acknowledgements
The authors thank Mr. Bernat Miguel, Statistician and Data Manager of the Colorectal Unit, University Hospital of Bellvitge and IDIBELL, for the statistical analysis.
Funding
There are no funding sources for the present observational study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Biondo, S., Gálvez, A., Ramírez, E. et al. Emergency surgery for obstructing and perforated colon cancer: patterns of recurrence and prognostic factors. Tech Coloproctol 23, 1141–1161 (2019). https://doi.org/10.1007/s10151-019-02110-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-019-02110-x