Abstract
Aims and objectives
Human epidermal growth factor receptor 2 (HER2)-low breast cancer (BC) is a new entity considered a biologically distinct subtype from HER2-zero BC. However, the importance of HER2 low expression on the activity of cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) remains unclear.
Methods/materials
We conducted a single-center retrospective study including hormone receptor-positive (HR +) /HER2- metastatic BC (mBC) patients treated with CDK4/6i plus endocrine treatment (ET) as first-line therapy. Clinical outcomes were analyzed according to HER2 expression.
Results
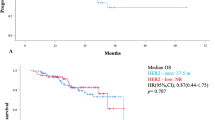
258 women were analyzed with a median follow-up of 25.4 months; 39.9% had HER2 low, and 60.1% had HER2 zero BC. Median progression-free survival (mPFS) in the HER2-low group was 27.6 months compared with 44.3 months in the HER2-zero group (p = 0.341). In patients receiving ribociclib, the mPFS in the HER2-low group was 24.2 months compared with 53.1 months in the HER2-zero group (multivariate-adjusted HR: 1.981, 95 Cl 1.094–3.586; p = 0.024). The survival probabilities at 24, 36 and 48 months for the HER2 low and HER2 zero groups were 82%, 69%, 69% and 83%, 75% and 69%, respectively (p = 0.336). Objective response rate (p = 0.179) and disease control rate (p = 0.338) did not significantly differ between HER-2-low and HER-2-zero groups.
Conclusions
The mPFS in the Her2-zero group was almost twice that of the Her2-low group, but the difference was not statistically significant. mPFS was significantly longer in the HER2-zero group compared to the HER2-low group in patients receiving ribociclib. More prospective studies are needed to understand the actual consequences of this biomarker.

Similar content being viewed by others
Data availability
Data supporting the findings of this study are available from the corresponding author upon request.
References
Basaran GA et al (2018) Ongoing unmet needs in treating estrogen receptor-positive/HER2-negative metastatic breast cancer. Cancer Treat Rev 63:144–155
Reinert T, Barrios CH (2017) Overall survival and progression-free survival with endocrine therapy for hormone receptor-positive, HER2-negative advanced breast cancer: review. Ther Adv Med Oncol 9(11):693–709
Hart CD et al (2015) Challenges in the management of advanced, ER-positive, HER2-negative breast cancer. Nat Rev Clin Oncol 12(9):541–552
Jin X et al (2023) Molecular classification of hormone receptor-positive HER2-negative breast cancer. Nat Genet 55(10):1696–1708
Finn RS, Aleshin A, Slamon DJ (2016) Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 18(1):17
Giuliano M, Trivedi MV, Schiff R (2013) Bidirectional crosstalk between the estrogen receptor and human epidermal growth factor receptor 2 signaling pathways in breast cancer: molecular basis and clinical implications. Breast Care (Basel) 8(4):256–262
Osborne CK et al (2005) Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res 11(2 Pt 2):865s–870s
Arpino G et al (2008) Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev 29(2):217–233
Modi S et al (2022) Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 387(1):9–20
Banerji U et al (2019) Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol 20(8):1124–1135
Modi S et al (2020) Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase ib study. J Clin Oncol 38(17):1887–1896
Wang J et al (2021) RC48-ADC a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: a pooled analysis of two studies. J Clin Oncol 39:1022–1022
Denkert C et al (2021) Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol 22(8):1151–1161
Schettini F et al (2021) Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 7(1):1
Agostinetto E et al (2021) HER2-low breast cancer: molecular characteristics and prognosis. Cancers (Basel) 13(11):2824. https://doi.org/10.3390/cancers13112824
Rossi V et al (2012) Moderate immunohistochemical expression of HER-2 (2+) without HER-2 gene amplification is a negative prognostic factor in early breast cancer. Oncologist 17(11):1418–1425
Gilcrease MZ et al (2009) Even low-level HER2 expression may be associated with worse outcome in node-positive breast cancer. Am J Surg Pathol 33(5):759–767
Jacot W et al (2021) Prognostic value of HER2-low expression in non-metastatic triple-negative breast cancer and correlation with other biomarkers. Cancers (Basel) 13(23):6059. https://doi.org/10.3390/cancers13236059
Gampenrieder SP et al (2021) Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res 23(1):112
Jiang C et al (2022) Clinical outcomes of de novo metastatic HER2-low breast cancer: a national cancer database analysis. NPJ Breast Cancer 8(1):135
Burris HA 3rd et al (2011) Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 29(4):398–405
Fehrenbacher L et al (2020) NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol 38(5):444–453
Gianni L et al (2010) Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 28(7):1131–1137
Sharaf B et al (2023) Differences in treatment outcomes between patients with HER2-low versus HER2-zero, hormone receptor-positive advanced-stage breast cancer treated with ribociclib. Breast Cancer (Dove Med Press) 15:541–548
Zattarin E et al (2023) Prognostic significance of HER2-low status in HR-positive/HER2-negative advanced breast cancer treated with CDK4/6 inhibitors. NPJ Breast Cancer 9(1):27
Bao KKH et al (2021) The association of ERBB2-low expression with the efficacy of cyclin-dependent kinase 4/6 inhibitor in hormone receptor-positive, ERBB2-negative metastatic breast cancer. JAMA Netw Open 4(11):e2133132
Bayona R et al (2022) Abstract P5–13-24: efficacy of first line CDK4/6 inhibitors in HER2-low vs HER2-zero, hormone receptor positive, HER2 negative metastatic breast cancer. Can Res 82:P5-13
Carlino F et al (2022) HER2-low status does not affect survival outcomes of patients with metastatic breast cancer (MBC) undergoing first-line treatment with endocrine therapy plus palbociclib: results of a multicenter. Retrosp Cohort Study Cancer (Basel) 14(20):4981. https://doi.org/10.3390/cancers14204981
Douganiotis G et al (2022) Prognostic significance of low HER2 expression in patients with metastatic hormone receptor-positive breast cancer treated with first line CDK4/6 inhibitors: a greek multicenter real-world data analysis. Cancer Diagn Progn 2(5):585–591
Lapuchesky LS et al (2022) CDK4/6 inhibitors outcomes in patients with advanced breast cancer based on HER2-low expression. J Clin Oncol 40:1056–1056. https://doi.org/10.1200/JCO.2022.40.16_suppl.1056
Shao Y et al (2022) HER2-low expression does not affect the clinical outcomes of metastatic breast cancer treated with CDK4/6 inhibitor: a real-world study. Front Endocrinol (Lausanne) 13:1000704
Caliskan Yildirim E et al (2023) The effect of low HER2 expression on treatment outcomes in metastatic hormone receptor positive breast cancer patients treated with a combination of a CDK4/6 inhibitor and endocrine therapy: a multicentric retrospective study. Breast 70:56–62
Cardoso F et al (2018) 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4) dagger. Ann Oncol 29(8):1634–1657
Spring LM et al (2020) Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet 395(10226):817–827
Hortobagyi GN et al (2022) Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med 386(10):942–950
Zhang G et al (2022) Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med 20(1):142
Osborne CK, Schiff R (2011) Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 62:233–247
Mazumder A, Shiao S, Haricharan S (2021) HER2 activation and endocrine treatment resistance in HER2-negative breast cancer. Endocrinology. https://doi.org/10.1210/endocr/bqab153
Eggemann H et al (2015) Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer 22(5):725–733
Kang S et al (2022) Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur J Cancer 176:30–40
Li Y et al (2021) In real life, low-level HER2 expression may be associated with better outcome in HER2-negative breast cancer: a study of the national cancer center. China Front Oncol 11:774577
de Calbiac O et al (2022) Comparison of management and outcomes in ERBB2-low vs ERBB2-zero metastatic breast cancer in France. JAMA Netw Open 5(9):e2231170
Won HS et al (2022) Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean breast cancer society. Breast Cancer Res 24(1):22
Tarantino P et al (2022) Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer 163:35–43
Miglietta F et al (2021) Author correction: evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer 7(1):149
Marchio C et al (2021) Evolving concepts in HER2 evaluation in breast cancer: heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol 72:123–135
Shah SP et al (2009) Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature 461(7265):809–813
Tanner M, Jarvinen P, Isola J (2001) Amplification of HER-2/neu and topoisomerase IIalpha in primary and metastatic breast cancer. Cancer Res 61(14):5345–5348
Cao N et al (2009) NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat Res 171(1):9–21
Wright C et al (1992) Relationship between c-erbB-2 protein product expression and response to endocrine therapy in advanced breast cancer. Br J Cancer 65(1):118–121
Kan S et al (2015) Gemcitabine treatment enhances HER2 expression in low HER2-expressing breast cancer cells and enhances the antitumor effects of trastuzumab emtansine. Oncol Rep 34(1):504–510
Wolff AC et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013
Wolff AC et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol 36(20):2105–2122
Marra A, Curigliano G (2019) Are all cyclin-dependent kinases 4/6 inhibitors created equal? NPJ Breast Cancer 5:27
Palbociclib for breast cancer (2018) Aust Prescr 41(4):127–128. https://doi.org/10.18773/austprescr.2018.029 (PMID: 30116084; PMCID: PMC6091774)
Fry DW et al (2004) Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 3(11):1427–1438
Gelbert LM et al (2014) Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs 32(5):825–837
Tripathy D, Bardia A, Sellers WR (2017) Ribociclib (LEE011): mechanism of action and clinical impact of this selective cyclin-dependent kinase 4/6 inhibitor in various solid tumors. Clin Cancer Res 23(13):3251–3262
Hein A et al (2021) Prognostic effect of low-level HER2 expression in patients with clinically negative HER2 status. Eur J Cancer 155:1–12
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the article and approved the submitted version. T.Ö, C.K and Ö.A designed the research, analyzed the data and drafted the paper. T.Ö, C.K, Ö.A, and İ.Ö were mainly responsible for data collection and analysis. T.Ö and C.K were primarily responsible for statistical analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The studies involving human participants were reviewed and approved by The ethics committee of Health Sciences University, Dr. Abdurrahman Yurtaslan Oncology Training and Research Hospital. According to national legislation and institutional standards, participation in this study did not require written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Önder, T., Ateş, Ö., Öner, İ. et al. Relationship between HER2-low status and efficacy of CDK4/6 inhibitors in advanced breast cancer: a real-world study. Int J Clin Oncol (2024). https://doi.org/10.1007/s10147-024-02528-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10147-024-02528-w