Abstract
Background
Recent advances in immune-checkpoint inhibitors (ICIs) have highlighted the need for effective management of immune-related adverse events (irAEs). This study aimed to conduct a systematic surveillance of real-world development of irAEs for understanding their characteristics and examine the prognostic impact of steroid use for these events.
Methods
We retrospectively investigated cancer patients treated with ICIs between 2014 and 2021 and collected information about irAEs throughout their development, management, and clinical outcomes.
Results
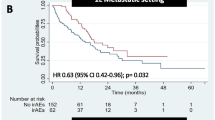
Overall, 458 patients (45.4%) developed 670 irAEs. The prevalence of irAEs varied by cancer type, but it was increased in regimens with longer treatment durations. Severe irAEs were more common in the nivolumab + ipilimumab and pembrolizumab + axitinib regimens. Patients who received steroids for irAEs at a dosage of < 2 mg/kg had comparable prognosis to those who did not receive steroids; however, patients who received methylprednisolone pulse therapy, primarily for severe pneumonitis and hepatitis, had shorter overall survival than those who did not receive steroids (7.8 versus 23.4 months, p = 0.016). Furthermore, methylprednisolone pulse therapy for irAEs was a poor prognostic factor in multivariate analysis (hazard ratio: 2.19, 95% confidence interval: 1.34–2.86, p < 0.001).
Conclusion
Steroid treatment for irAE does not affect prognosis and should thus be used promptly to control inflammation. However, pulse therapy for severe cases is a poor prognostic factor, and early detection remains the key to managing such irAEs. The irAE characteristics in each regimen should be clarified to establish and provide more sophisticated irAE management, and the current findings will be beneficial to this goal.






Similar content being viewed by others
References
Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint blockade in cancer therapy. J Clin Oncol 33:1974–1982
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355
de Miguel M, Calvo E (2020) Clinical challenges of immune checkpoint inhibitors. Cancer Cell 38:326–333
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378:158–168
Martins F, Sofiya L, Sykiotis GP et al (2019) Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 16:563–580
Johnson DB, Nebhan CA, Moslehi JJ et al (2022) Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol 19(4):254–267
Xu C, Chen Y-P, Du X-J et al (2018) Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ 363:k4226
Brahmer JR, Abu-Sbeih H, Ascierto PA et al (2021) Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 9:e002435
Schneider BJ, Naidoo J, Santomasso BD et al (2021) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol 39:4073–4126
Haanen J, Obeid M, Spain L et al (2022) Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 33:1217–1238
Zamami Y, Niimura T, Okada N et al (2019) Factors associated with immune checkpoint inhibitor-related myocarditis. JAMA Oncol 5:1635–1637
Raschi E, Gatti M, Gelsomino F et al (2020) Lessons to be learnt from real-world studies on immune-related adverse events with checkpoint inhibitors: a clinical perspective from pharmacovigilance. Target Oncol 15:449–466
Reynolds KL, Arora S, Elayavilli RK et al (2021) Immune-related adverse events associated with immune checkpoint inhibitors: a call to action for collecting and sharing clinical trial and real-world data. J Immunother Cancer 9:e002896
Bruera S, Suarez-Almazor ME (2022) The effects of glucocorticoids and immunosuppressants on cancer outcomes in checkpoint inhibitor therapy. Front Oncol 12:928390
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48:452–458
Xing P, Zhang F, Wang G et al (2019) Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. J Immunother Cancer 7:341
Shulgin B, Kosinsky Y, Omelchenko A et al (2020) Dose dependence of treatment-related adverse events for immune checkpoint inhibitor therapies: a model-based meta-analysis. Oncoimmunology 9:1748982
Rini BI, Plimack ER, Stus V et al (2019) Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116–1127
Grünwald V, Voss MH, Rini BI et al (2020) Axitinib plus immune checkpoint inhibitor: evidence- and expert-based consensus recommendation for treatment optimisation and management of related adverse events. Br J Cancer 123:898–904
Rini BI, Atkins MB, Plimack ER et al (2022) Characterization and management of treatment-emergent hepatic toxicity in patients with advanced renal cell carcinoma receiving first-line pembrolizumab plus axitinib. results from the KEYNOTE-426 Trial. Eur Urol Oncol 5:225–234
Freeman-Keller M, Kim Y, Cronin H et al (2016) Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 22:886–894
Haratani K, Hayashi H, Chiba Y et al (2018) Association of immune-related adverse events with nivolumab efficacy in non–small-cell lung cancer. JAMA Oncol 4:374–378
Matsuo M, Yasumatsu R, Masuda M et al (2020) Relationship between immune-related adverse events and the long-term outcomes in recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab. Oral Oncol 101:104525
Ruste V, Goldschmidt V, Laparra A et al (2021) The determinants of very severe immune-related adverse events associated with immune checkpoint inhibitors: a prospective study of the French REISAMIC registry. Eur J Cancer 158:217–224
Matsukane R, Watanabe H, Minami H et al (2021) Continuous monitoring of neutrophils to lymphocytes ratio for estimating the onset, severity, and subsequent prognosis of immune related adverse events. Sci Rep 11:1324
Arbour KC, Mezquita L, Long N et al (2018) Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 36:2872–2878
Scott SC, Pennell NA (2018) Early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol 13:1771–1775
Fucà G, Galli G, Poggi M et al (2019) Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 4:e000457
Ricciuti B, Dahlberg SE, Adeni A et al (2019) Immune checkpoint inhibitor outcomes for patients with non–small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol 37:1927–1934
Skribek M, Rounis K, Afshar S et al (2021) Effect of corticosteroids on the outcome of patients with advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Eur J Cancer 145:245–254
De Giglio A, Mezquita L, Auclin E et al (2020) Impact of intercurrent introduction of steroids on clinical outcomes in advanced non-small-cell lung cancer (NSCLC) patients under immune-checkpoint inhibitors (ICI). Cancers 12:2827
Faje AT, Lawrence D, Flaherty K et al (2018) High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 124:3706–3714
Eggermont AMM, Kicinski M, Blank CU et al (2020) Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol 6:519–527
Horvat TZ, Adel NG, Dang T-O et al (2015) Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol 33:3193–3198
Mouri A, Kaira K, Yamaguchi O et al (2021) Effect of systemic steroid use for immune-related adverse events in patients with non-small cell lung cancer receiving PD-1 blockade drugs. J Clin Med Res 10:3744
Martins F, Sykiotis GP, Maillard M et al (2019) New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol 20:e54–e64
Wang H, Zhou J, Guo X et al (2020) Use of glucocorticoids in the management of immunotherapy-related adverse effects. Thorac Cancer 11:3047–3052
Allouchery M, Lombard T, Martin M et al (2020) Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J Immunother Cancer 8:e001622
Dolladille C, Ederhy S, Sassier M et al (2020) Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol 6:865–871
Zhao Q, Zhang J, Xu L et al (2021) Safety and efficacy of the rechallenge of immune checkpoint inhibitors after immune-related adverse events in patients with cancer: a systemic review and meta-analysis. Front Immunol 12:730320
Acknowledgements
We appreciate the physicians and medical workers who managed the ICI and irAEs. We would especially like to thank all the patients involved in this study. This work was partly supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI [grant numbers JP20H01058 to RM] and the Foundation for Promotion of Cancer Research in Japan for RM.
Author information
Authors and Affiliations
Contributions
R.M., K.S. and K.H. conceived and planned the study. R.M., K.M., S.N. and H.M. contributed the retrospective data collection from electric records. R.M. analyzed the data. R.M., K.S., K.H., H.W., T.H., N.E. and I.I. contributed to the interpretation of the results. R.M. took the lead in writing the manuscript. All the authors provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No author has any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Matsukane, R., Suetsugu, K., Hata, K. et al. Systematic surveillance of immune-related adverse events in clinical practice and impact of subsequent steroid medication on survival outcomes. Int J Clin Oncol 28, 860–871 (2023). https://doi.org/10.1007/s10147-023-02349-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02349-3