Abstract
Purpose
To investigate the pain-relieving effect and safety of three different doses of 188Re-hydroxyethylidine diphosphonate (HEDP) in patients with lung cancer and bone metastases.
Methods
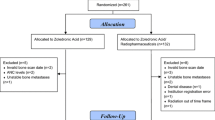
For this randomised, phase 2 and multicenter trial, we enrolled patients with lung carcinoma and multifocal bone metastases and excluded patients who had received bisphosphonates or external-beam radiotherapy within the previous 4 weeks. Fifty-four patients were randomized to receive a single injection of 188Re-HEDP, at doses of 30, 40 or 50 MBq/kg (interval, 12 weeks). Patients were followed-up by assessment of numerical rating scale (NRS) score, global quality of life (QOL) score and adverse events (AEs). ANOVA analysis, Chi-Squared test and LSD-t test were used in this study.
Results
Significantly decreased NRS scores relative to baseline were observed in 40 MBq/kg group (Week 0 vs. Week 12: 6.0 ± 1.4 vs. 4.8 ± 2.5, P = 0.033) and 50 MBq/kg group (Week 0 vs. Week 12: 5.5 ± 1.5 vs. 4.5 ± 2.9, P = 0.046). Significant change of global QOL score from baseline was observed in 40 MBq/kg group at week 8 (global QOL score: P = 0.024, pain score: P = 0.041) and 50 MBq/kg group (pain score: P = 0.021) at week 12. No patients withdrew trial because of AEs in three groups.
Conclusions
188Re-HEDP at dose of 40 and 50 MBq/kg was generally effective to alleviate pain and improve QOL in lung cancer patients with painful bone metastases. 188Re-HEDP was safe and well-tolerated.



Similar content being viewed by others
References
Lortet-Tieulent J, Soerjomataram I, Ferlay J et al (2014) International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer 84(1):13–22. https://doi.org/10.1016/j.lungcan.2014.01.009
Mao Y, Yang D, He J, Krasna MJ (2016) Epidemiology of lung cancer. Surg Oncol Clin N Am 25(3):439–445. https://doi.org/10.1016/j.soc.2016.02.001
Nanavaty P, Alvarez MS, Alberts WM (2014) Lung cancer screening: advantages, controversies, and applications. Cancer Control 21(1):9–14. https://doi.org/10.1177/107327481402100102
Santini D, Barni S, Intagliata S et al (2016) Natural history of non-small-cell lung cancer with bone metastases (vol 5, 18670, 2015). Sci Rep 6:18670. https://doi.org/10.1038/srep22205
Nilsson S, Strang P, Aksnes AK et al (2012) A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer 48(5):678–686. https://doi.org/10.1016/j.ejca.2011.12.023
Parker C, Zhan L, Cislo P et al (2017) Effect of radium-223 dichloride (Ra-223) on hospitalisation: an analysis from the phase 3 randomised alpharadin in symptomatic prostate cancer patients (ALSYMPCA) trial. Eur J Cancer 71:1–6. https://doi.org/10.1016/j.ejca.2016.10.020
Kasalický J, Krajská V (1998) The effect of repeated strontium-89 chloride therapy on bone pain palliation in patients with skeletal cancer metastases. Eur J Nucl Med 25(10):1362–1367. https://doi.org/10.1007/s002590050309
Oosterhof GO, Roberts JT, de Reijke TM et al (2003) Strontium(89) chloride versus palliative local field radiotherapy in patients with hormonal escaped prostate cancer: a phase III study of the European Organisation for Research and Treatment of Cancer, Genitourinary Group. Eur Urol 44(5):519–526. https://doi.org/10.1016/s0302-2838(03)00364-6
Porter AT, McEwan AJ, Powe JE et al (1993) Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys 25(5):805–813. https://doi.org/10.1016/0360-3016(93)90309-j
Knapp FF Jr (1998) Rhenium-188–a generator-derived radioisotope for cancer therapy. Cancer Biother Radiopharm 13(5):337–349. https://doi.org/10.1089/cbr.1998.13.337
Lambert B, de Klerk JM (2006) Clinical applications of 188Re-labelled radiopharmaceuticals for radionuclide therapy. Nucl Med Commun 27(3):223–229. https://doi.org/10.1097/00006231-200603000-00004
Lange R, Overbeek F, de Klerk JM et al (2016) Treatment of painful bone metastases in prostate and breast cancer patients with the therapeutic radiopharmaceutical rhenium-188-HEDP Clinical benefit in a real-world study. Nuklearmedizin 55(5):188–195. https://doi.org/10.3413/Nukmed-0828-16-05
Cheng A, Chen S, Zhang Y et al (2011) The tolerance and therapeutic efficacy of rhenium-188 hydroxyethylidene diphosphonate in advanced cancer patients with painful osseous metastases. Cancer Biother Radiopharm 26(2):237–244. https://doi.org/10.1089/cbr.2010.0873
Liepe K, Kropp J, Runge R et al (2003) Therapeutic efficiency of rhenium-188-HEDP in human prostate cancer skeletal metastases. Br J Cancer 89(4):625–629. https://doi.org/10.1038/sj.bjc.6601158
Zhang H, Tian M, Li S et al (2003) Rhenium-188-HEDP therapy for the palliation of pain due to osseous metastases in lung cancer patients. Cancer Biother Radiopharm 18(5):719–726. https://doi.org/10.1089/108497803770418265
Lin WY, Lin CP, Yeh SJ et al (1997) Rhenium-188 hydroxyethylidene diphosphonate: a new generator-produced radiotherapeutic drug of potential value for the treatment of bone metastases. Eur J Nucl Med 24(6):590–595. https://doi.org/10.1007/bf00841394
Savio E, Gaudiano J, Robles AM et al (2001) Re-HEDP : pharmacokinetic characterization, clinical and dosimetric evaluation in osseous metastatic patients with two levels of radiopharmaceutical dose. BMC Nucl Med 1(1):2. https://doi.org/10.1186/1471-2385-1-2
Liepe K, Hliscs R, Kropp J et al (2000) Rhenium-188-HEDP in the palliative treatment of bone metastases. Cancer Biother Radiopharm 15(3):261–265. https://doi.org/10.1089/108497800414356
Palmedo H, Manka-Waluch A, Albers P et al (2003) Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: tandomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. J Clin Oncol 21(15):2869–2875. https://doi.org/10.1200/jco.2003.12.060
Akobeng AK (2016) Understanding type I and type II errors, statistical power and sample size. Acta Paediatr 105(6):605–609. https://doi.org/10.1111/apa.13384
Acknowledgements
The authors thank all study participants for their great effort.
Funding
This study was supported by the grants from the Ministry of Science and Technology of China (2017YFC0113300), the National Natural Science Fund of China (No. 81571706) and China Scholarship Council (CSC, No. 202006100180).
Author information
Authors and Affiliations
Contributions
Xingdang Liu is corresponding author who was coordinating investigator (COI) in this study. Ping Chen is first author who was co-investigator (CI) in this study. Yiwei Wu, Zengli Liu, Changjing Zuo, Zhongwei Lv, Yingjian Zhang and Biao Li were principal investigator (PI) who screened patients into clinical trial. Jun Li, Jicong Gui, Congjin Liu, Yuankai Wang, Guangming Zhang, Dayu Kuai were sub-investigator (SI) who recorded and analyzed data.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Chen, P., Li, J., Gui, J. et al. Efficacy and safety of 188Re-HEDP in lung cancer patients with bone metastases: a randomized, multicenter, multiple-dose phase IIa study. Int J Clin Oncol 26, 1212–1220 (2021). https://doi.org/10.1007/s10147-021-01906-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01906-y