Abstract
Background
Although regorafenib or trifluridine/tipiracil (FTD/TPI) has been recognized as a later-line standard treatment in patients with metastatic colorectal cancer (mCRC), not all patients have beneficial outcomes. This study aimed to develop a prognostic scoring system for evaluating the overall survival (OS) benefit.
Methods
Patients included in the REGOTAS study, which comprised 489 patients (regorafenib group: 199; FTD/TPI group: 290 patients), were evaluated. OS was analyzed using multivariate Cox proportional model. The prognostic score was calculated using the worst four individual factors weighted by hazard ratio, and the total scores were categorized as low-, moderate-, and high-OS benefit.
Results
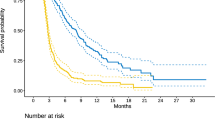
The worst four factors in the regorafenib group were AST > 40 IU/dL (point, + 3), CRP ≥ 1.0 mg/dL (+ 2), number of metastatic organ site ≥ 3 (+ 2), and duration from initiation of 1st-line chemotherapy < 18 months (+ 2), while they were AST (+ 2), CRP (+ 2), CA19-9 > 37.0 U/mL (+ 2), and ECOG PS ≥ 1 (+ 2) in the FTD/TPI group. These corresponded to a total prognostic score of > 5, 2–4, and 0 points in the regorafenib group and 8, 2–6, and 0 points in the FTD/TPI group. The median OS in the low, moderate, and high OS benefit group was 3.3 (95% CI 3.0–3.7), 8.1 (95% CI 6.4–9.7), and 12.6 months (95% CI 10.6–14.6) in the regorafenib group and 2.8 (95% CI 2.0–3.5), 7.5 (95% CI 6.6–8.3), and 15.4 months (95% CI 9.7–21.2) in the FTD/TPI group.
Conclusion
These prognostic scores are useful for identifying patients with mCRC who will obtain survival benefits from these drugs.


Similar content being viewed by others
References
Modest DP, Pant S, Sartore-Bianchi A (2019) Treatment sequencing in metastatic colorectal cancer. Eur J Cancer 109:70–83
Grothey A, Van Cutsem E, Sobrero A et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381(9863):303–312
Mayer RJ, Van Cutsem E, Falcone A et al (2015) Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 372(20):1909–1919
Sueda T, Sakai D, Kudo T et al (2016) Efficacy and safety of regorafenib or TAS-102 in patients with metastatic colorectal cancer refractory to standard therapies. Anticancer Res 36(8):4299–4306
Masuishi T, Taniguchi H, Hamauchi S et al (2017) Regorafenib versus trifluridine/tipiracil for refractory metastatic colorectal cancer: a retrospective comparison. Clin Colorectal Cancer 16(2):e15–e22
Unseld M, Drimmel M, Siebenhuner A et al (2018) Optimizing treatment sequence for late-line metastatic colorectal cancer patients using trifluridine/tipiracil and regorafenib. Clin Colorectal Cancer 17(4):274–279
Moriwaki T, Fukuoka S, Taniguchi H et al (2018) Propensity score analysis of regorafenib versus trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy (REGOTAS): a Japanese Society for Cancer of the Colon and Rectum Multicenter Observational Study. Oncologist 23(1):7–15
Yamaguchi K, Komatsu Y, Satoh T et al (2019) Large-scale, prospective observational study of regorafenib in Japanese patients with metastatic colorectal cancer in a real-world clinical setting. Oncologist 24:e450–e457
Cremolini C, Rossini D, Martinelli E et al (2018) Trifluridine/tipiracil (TAS-102) in refractory metastatic colorectal cancer: a multicenter register in the frame of the Italian Compassionate Use Program. Oncologist 23(10):1178–1187
Shibutani M, Nagahara H, Fukuoka T et al (2019) Prognostic significance of the C-reactive protein-to-albumin ratio in patients with metastatic colorectal cancer treated with trifluridine/thymidine phosphorylase inhibitor as later-line chemotherapy. Anticancer Res 39(2):1051–1057
Tsuchihashi K, Ito M, Moriwaki T et al (2018) Role of predictive value of the modified Glasgow Prognostic Score for later-line chemotherapy in patients with metastatic colorectal cancer. Clin Colorectal Cancer 17(4):e687–e697
Adenis A, de la Fouchardiere C, Paule B et al (2016) Survival, safety, and prognostic factors for outcome with regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 16:412
Riedl JM, Posch F, Moik F et al (2017) Inflammatory biomarkers in metastatic colorectal cancer: prognostic and predictive role beyond the first line setting. Oncotarget 8(56):96048–96061
Skuja E, Gerina-Berzina A, Hegmane A et al (2018) Duration of previous treatment as a prognostic factor in metastatic colorectal cancer treated with trifluridine/tipiracil. Mol Clin Oncol 8(5):699–702
Song A, Eo W, Lee S (2015) Comparison of selected inflammation-based prognostic markers in relapsed or refractory metastatic colorectal cancer patients. World J Gastroenterol 21(43):12410–12420
Acknowledgements
This work was supported by funding from the JSCCR. We would like to thank Editage (https://www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Atsuo Takashima received a research grant from Takeda, MSD, LSK, and Sumitomo Dainippon. Kentaro Yamazaki received honoraria from Taiho and Bayer, and a research grant from Taiho. Taito Esaki received honoraria from Lilly, and a research grant from Astellas, MSD, Dai-ichi-Sankyo, Ono, Merck Serono, Dainippon Sumitomo, Novartis, Taiho, and Lilly, and scholarship donations from Ono. Naotoshi Sugimoto received a research grant from MSD, Ono, Astellas, Dai-ichi Sankyo. Eiji Oki received honoraria from Taiho and Bayer. Yoshito Komatsu received honoraria from Lilly, Taiho, Chugai, Takeda, Bayer, Bristol Myers, Sanofi, Merck Serono, Yakult, and MSD, and a research grant from NanoCarrier, Baxter, Linical, QuintilesIMS, Sysmex, Mediscience, Ono, Dainippon Sumitomo, MSD, Taiho, Dai-ichi-Sankyo, and Yakult, and scholarship donations from Taiho, Chugai, Kyowa Kirin, Takeda, and Ono. Akihito Tsuji received honoraria from Taiho, Chugai, Takeda, Merck Serono, Lilly, Sanofi, and Bristol, and a research grant from Bristol Meyer, Ono, Taiho, and Sanofi. Daisuke Sakai received honoraria from Chugai, and a research grant from Lilly, Ono, Dai-ichi-Sankyo, and Astellas, and endowed chairs from Yakult, Chugai, and Ono. Hideki Ueno received a research grant from Taiho, Yakult, Bayer, Chugai, and Sysmex. Yasuhiro Shimada received a research grant from Taiho, MSD, and Lilly. All other authors declare no potential conflict of interest.
Ethical approval
The present study was approved from the Ethics Committee of the JSCCR. The requirement for informed consent was waived because of the retrospective design of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Moriwaki, T., Fukuoka, S., Masuishi, T. et al. Prognostic scores for evaluating the survival benefit of regorafenib or trifluridine/tipiracil in patients with metastatic colorectal cancer: an exploratory analysis of the REGOTAS study. Int J Clin Oncol 25, 614–621 (2020). https://doi.org/10.1007/s10147-019-01600-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01600-0