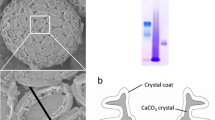
Abstract
The effects of Ca2+ and Mg2+ on cellular growth and calcification in Pleurochrysis haptonemofera were investigated. In the presence of a normal concentration of Mg2+, coccolith-bearing cells (C-cells) required more than 0.5 mM Ca2+ for growth, while naked cells could grow even with 0.5 mM Ca2+. The calcification rate of C-cells, which was determined using decalcified cells, was significantly repressed with less than or equal to 0.5 mM Ca2+. Although the calcification rate did not change so much with 5–30 mM Ca2+, it decreased with higher concentrations of Ca2+, as well as C-cell-specific growth repression. Under these conditions, Ca2+ affected the rate of coccolith formation, but neither the coccolith morphology nor total amounts and ratios of divalent cations and acidic polysaccharides (Ph-PS-1, -2, and -3) were included in coccoliths. These findings suggest that sufficient calcification is required for the division of C-cells. Under low Ca2+ and high Mg2+ conditions, coccoliths with an abnormal morphology, having immature shield elements, were synthesized. Composition analysis of the coccoliths revealed high Mg/Ca and low Ph-PS-2/(Ph-PS-1 and -3) ratios, as compared with those under low Ca2+ and normal Mg2+ conditions, suggesting that the abnormal morphology is due to a change in the crystal type and/or acidic polysaccharide composition.








Similar content being viewed by others
References
Berges JA, Franklin DJ, Harrison PJ (2001) Evolution of an artificial seawater medium improvements in enriched seawater, artificial water over the last two decades. J Phycol 37:1138–1145
Blackwelder PL, Weiss RE, Wilbur KM (1976) Effects of calcium, strontium, and magnesium on the coccolithophorid Cricosphaera (Hymenomonas) carterae. I. Calcification. Mar Biol 34:11–16
Boney AD, Burrows A (1966) Experimental studies on the benthic phases of Haptophyceae. I. Effects of some experimental conditions on the release of coccolithophorids. J Mar Biol Assoc UK 46:295–319
Borman AH, de Jong EW, Huizinga M, Kok DJ, Westbroek P, Bosch L (1982) The role in CaCO3 crystallization of an acid Ca2+-binding polysaccharide associated with coccoliths of Emiliania huxleyi. Eur J Biochem 129:179–183
Davis KJ, Dove PM, de Yoreo JJ (2000) The role of Mg2+ as an impurity in calcite growth. Science 290:1134–1137
de Jong EW, Bosch L, Westbroek P (1976) Isolation and characterization of a Ca2+-binding polysaccharide associated with coccoliths of Emiliania huxleyi (Lohmann) Kamptner. Eur J Biochem 70:611–621
Fujiwara S, Hirokawa Y, Takatsuka Y, Suda K, Asamizu E, Takayanagi T, Shibata D, Tabata S, Tsuzuki M (2007) Gene expression profiling of coccolith-bearing cells and naked cells in haptophyte Pleurochrysis haptonemofera with a cDNA macroarray system. Mar Biotechnol 9:550–560
Green MR, Pastewka JV, Peacock A (1973) Differential staining of phosphoproteins on polyacrylamide gels with a cationic carbocyanine dye. Anal Biochem 56:43–51
Herfort L, Loste E, Meldrum F, Thake B (2004) Structural and physiological effects of calcium and magnesium in Emiliania huxleyi (Lohmann) Hay and Mohler. J Struct Biol 148:307–314
Hirokawa H, Fujiwara S, Tsuzuki M (2005) Three types of acidic polysaccharides associated with coccolith of Pleurochrysis haptonemofera: comparison of the biochemical characteristics with those of P. carterae and analysis using fluorescein-isothiocyanate-labeled lectins. Mar Biotechnol 7:634–644
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Marsh ME (1999) Coccolith crystals of Pleurochrysis carterae: crystallographic faces, organization and development. Protoplasma 207:54–66
Marsh ME (2003) Regulation of CaCO3 formation in coccolithophores. Comp Biochem and Physiol B 136:743–754
Marsh ME, Dickinson DP (1997) Polyanion-mediated mineralization—mineralization in coccolithophore (Pleurochrysis carterae) variants which do not express PS2, the most abundant and acidic mineral-associated polyanion in wild-type cells. Protoplasma 199:9–17
Marsh ME, Chang DK, King GC (1992) Isolation and characterization of a novel acidic polysaccharide containing tartrate and glyoxylate residues from the mineralized scales of a unicellular coccolithophorid alga Pleurochrysis carterae. J Biol Chem 267:20507–20512
Marsh ME, Ridall AL, Azadi P, Duke PJ (2002) Galacturonomannan and Golgi-derived membrane linked to growth and shaping of biogenic calcite. J Struct Biol 139:39–45
Okazaki M (1992) Algal calcification: its contribution to the “CO2 problem”. Korean J Phycol 7:147–154
Ozaki N, Sakuda S, Nagasawa H (2001) Isolation and some characterization of an acidic polysaccharide with anti-calcification activity from coccoliths of a marine alga, Pleurochrysis carterae. Biosci Biotechnol Biochem 65:2330–2333
Ozaki N, Sakuda S, Nagasawa H (2007) A novel highly acidic polysaccharide with inhibitory activity on calcification from the calcified scale “coccolith” of a coccolithophorid alga, Pleurochrysis haptonemofera. Biochem Biophys Res Commun 357:1172–1176
Paasche E (2002) A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification–photosynthesis interactions. Phycologia 40:503–529
Patton J, Reeder W (1956) New indicator for titration of calcium with (ethylenedinitrilo) tetraacetate. Anal Chem 28:1026–1028
Satoh M, Iwamoto K, Suzuki I, Shiraiwa Y (2009) Cold stress stimulates intracellular calcification by the coccolithophore, Emiliania huxleyi (Haptophyceae) under phosphate-deficient conditions. Mar Biotechnol 11:327–333
Shiraiwa Y (2003) Physiological regulation of carbon fixation in the photosynthesis and calcification of coccolithophorids. Comp Biochem Physiol B Biochem Mol Biol 136:775–783
Sikes CS, Wilbur KM (1980) Calcification by coccolithophorids: effects of pH and Sr. J Phycol 16:433–436
Takahashi J, Fujiwara S, Kikyo M, Hirokawa Y, Tsuzuki M (2002) Discrimination of the cell surface of the coccolithophorid Pleurochrysis haptonemofera from light scattering and fluorescein-isothiocyanate-labeled lectin staining measured by flow cytometry. Mar Biotechnol 4:94–101
Takayanagi T, Hirokawa Y, Yamamoto M, Ohki T, Fujiwara S, Tsuzuki M (2007) Protoplast formation of the coccolithophorid Pleurochrysis haptonemofera in hypoosmotic K+ solution: shedding of the coccosphere and regrowth of the protoplast in normal medium. Mar Biotechnol 9:56–65
Weiss RE, Blackwelder PL, Wilbur KM (1976) Effects of calcium, strontium, and magnesium on the coccolithophorid Cricosphaera (Hymenomonas) carterae. II. Cell division. Mar Biol 34:17–22
Young JR, Didymus JM, Bown PR, Prins B, Mann S (1992) Crystal assembly and phylogenetic evolution in heterococcoliths. Nature 356:516–518
Acknowledgements
The authors thank Drs. I. Inouye and M. Kawachi of Tsukuba University, Japan, for kindly providing the P. haptonemofera cells, for Mr. K. Suda and Drs. E. Asamizu, D. Shibata, and S. Tabata of Kazusa DNA Research Institute, Japan, for kindly supporting cDNA macroarray analyses, and Messrs. S. Miyashita and Y. Arai and Drs. T. Kaise and T. Uchida of Tokyo University of Pharmacy and Life Sciences, Japan, for the helpful support. This work was supported by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture, Japan (20570059) and the Promotion and Mutual Aid Corporation for Private Schools.
Author information
Authors and Affiliations
Corresponding author
Additional information
Fumio Katagiri and Yukiko Takatsuka contributed equally to this work.
Rights and permissions
About this article
Cite this article
Katagiri, F., Takatsuka, Y., Fujiwara, S. et al. Effects of Ca and Mg on Growth and Calcification of the Coccolithophorid Pleurochrysis haptonemofera: Ca Requirement for Cell Division in Coccolith-Bearing Cells and for Normal Coccolith Formation with Acidic Polysaccharides. Mar Biotechnol 12, 42–51 (2010). https://doi.org/10.1007/s10126-009-9198-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-009-9198-x