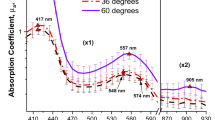
Abstract
There is a pressing need for monitoring cancerous tissue response to laser therapy. In this work, we evaluate the viability of elastic scattering spectroscopy (ESS) to monitor malignant transformations and effects of laser therapy of induced skin cancer in a hamster model. Skin tumors were induced in 35 mice, half of which were irradiated with 980 nm laser diode. Physiological and morphological transformations in the tumor were monitored over a period of 36 weeks using elastic scattering spectroscopy, in the near infrared window. Analytical model for light scattering was used to derive scattering optical properties for both transformed tissue and laser-treated cancer. The tissue scattering over the wavelength range (700–950 nm) decreased remarkably as the carcinogen-induced tissue transformed towards higher stages. Conversely, reduced scattering coefficient noticeably increased with increasing the number of laser irradiation sessions for the treated tumors. The relative changes in elastic scattering signal for transformed tissue were significantly different (p < .05). Elastic scattering signal intensity for laser-treated tissue was also significantly different (p < .05). Reduced scattering coefficient of treated tissue exhibited nearly 80% recovery of its normal skin value at the end of the experiment, and the treatment outcome could be improved by adjusting the number of sessions, which we can predict through spectroscopic optical feedback. This study demonstrates that ESS can quantitatively provide functional information that closely corresponds to the degree of pathologic transformation. ESS may well be a viable technique to optimize systemic melanoma and non-melanoma skin cancer treatment based on noninvasive tumor response.











Similar content being viewed by others
References
McCarthy K, Pearson K, Fulton R, Hewitt J (2012) Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev 12:CD008368
Rydzewska L, Tierney J, Vale CL, Symonds PR (2012) Neoadjuvant chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database Syst Rev 12:CD007406
Ueda S, Roblyer D, Cerussi A et al (2012) Baseline tumor oxygen saturation correlates with a pathologic complete response in breast cancer patients undergoing neoadjuvant chemotherapy. Cancer Res 72:4318–4328
Garland ML, Vather R, Bunkley N et al (2014) Clinical tumour size and nodal status predict pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Int J Color Dis 29:301–307
Jiang S, Pogue BW, Kaufman PA et al (2014) Predicting breast tumor response to neoadjuvant chemotherapy with diffuse optical spectroscopic tomography prior to treatment. Clin Cancer Res 20:6006–6015
Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49:6449–6465
Lehtio K, Eskola O, Viljanen T et al (2004) Imaging perfusion and hypoxia with PET to predict radiotherapy response in head-and-neck cancer. Int J Radiat Oncol Biol Phys 59:971–982
Jacobson O, Chen X (2013) Interrogating tumor metabolism and tumor microenvironments using molecular positron emission tomography imaging. Theranostic approaches to improve therapeutics. Pharmacol Rev 65:1214–1256
DeVries AF, Kremser C, Hein PA et al (2003) Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys 56:958–965
Hermans R, Lambin P, Van der Goten A et al (1999) Tumoural perfusion as measured by dynamic computed tomography in head and neck carcinoma. Radiother Oncol 53:105–111
Preda L, Calloni SF, Moscatelli ME et al (2014) Role of CT perfusion in monitoring and prediction of response to therapy of head and neck squamous cell carcinoma. Biomed Res Int 2014:917150
Anderson H, Price P, Blomley M et al (2001) Measuring changes in human tumour vasculature in response to therapy using functional imaging techniques. Br J Cancer 85:1085–1093
Turani Z, Fatemizadeh E, Blumetti T, et al (2019) Optical radiomic signatures derived from optical coherence tomography images improve identification of melanoma. Cancer Res 79(8):2021-2030
Avanaki MRN, Podoleanu AG, Schofield JB, Jones C, Sira M, Liu Y, Hojjat A (2013) Quantitative evaluation of scattering in optical coherence tomography skin images using the extended Huygens–Fresnel theorem. Appl Opt 52:1574–1580
Hafez R, Hamadah O, Bachir W (2015) Mapping of healthy oral mucosal tissue using diffuse reflectance spectroscopy: ratiometric-based total hemoglobin comparative study. Lasers Med Sci 30:2135 https://doi.org/10.1007/s10103-015-1765-y
Mourant JR, Freyer JP, Hielscher AH et al (1998) Mechanisms of light scattering from biological cells relevant to noninvasive optical-tissue diagnostics. Appl Opt 37:3586–3593
Mourant JR, Fuselier T, Boyer J et al (1997) Predictions and measurements of scattering and absorption over broad wavelength ranges in tissue phantoms. Appl Opt 36:949–957
Cheung C, Culver JP, Takahashi K et al (2001) In vivo cerebrovascular measurement combining diffuse near-infrared absorption and correlation spectroscopies. Phys Med Biol 46:2053–2065
Laughney AM, Krishnaswamy V, Rizzo EJ et al (2012) Scatter spectroscopic imaging distinguishes between breast pathologies in tissues relevant to surgical margin assessment. Clin Cancer Res 18:6315–6325
Sugimura T (1986) Studies on environmental chemical carcinogenesis in Japan. Science 233:312 Academic OneFile
SHubik P, Pietra G, Dellaporta G (1960) Studies of skin carcinogenesis in the Syrian golden hamster. Cancer Res 20:100–105
Shurrab K, Kochaji N, Bachir W (2017) Development of temperature distribution and light propagation model in biological tissue irradiated by 980 nm laser diode and using COMSOL simulation. J Laser Med Sci 8(3):118–122
Jacques SL (2013) Optical properties of biological tissues: a review. Phys Med Biol 58(11):R37–R61. https://doi.org/10.1088/0031-9155/58/11/r37
Rajaram N, Aramil TJ, Lee K, Reichenberg JS, Nguyen TH, Tunnell JW (2010) Design and validation of a clinical instrument for spectral diagnosis of cutaneous malignancy. Appl Opt 49(2):142–152
Rajaram N, Reichenberg JS, Migden MR, Nguyen TH, Tunnell JW (2010) Pilot clinical study for quantitative spectral diagnosis of non-melanoma skin cancer. Lasers Surg Med 42(10):716–727
Lim L, Nichols B, Migden MR, Rajaram N, Reichenberg JS, Markey MK, Ross MI, Tunnell JW (2014) Clinical study of noninvasive in vivo melanoma and nonmelanoma skin cancers using multimodal spectral diagnosis. J Biomed Opt 19:117003
Sharma M, Marple E, Reichenberg J, Tunnell JW (2014) Design and characterization of a novel multimodal fiber-optic probe and spectroscopy system for skin cancer applications. Rev Sci Instrum 85:083101
Boone MALM, Suppa M, Dhaenens F et al (2016) In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res 308:7. https://doi.org/10.1007/s00403-015-1608-5
Shurrab K, Kochaji N, Bachir W (2019) Effect of laser irradiation on the progression of skin cancer using carcinogen among hamsters. Iran J Med Phys 16(4):314–318
Acknowledgments
The authors gratefully acknowledge extend their gratitude to all colleagues at Damascus University and Higher Institute for Laser Research and Applications who cooperated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were approved by institutional ethical, according to Damascus University ethical committee decision no. 3164
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We state that this is an original article that has not been previously published, and that it is not simultaneously under consideration by any other journal
Rights and permissions
About this article
Cite this article
Shurrab, K., Kochaji, N. & Bachir, W. Elastic scattering spectroscopy for monitoring skin cancer transformation and therapy in the near infrared window. Lasers Med Sci 35, 701–708 (2020). https://doi.org/10.1007/s10103-019-02894-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02894-2