Abstract
Acute respiratory infections (ARIs) are caused by a variety of microorganisms. Of all ARIs, 80% are caused by viruses such as human respiratory syncytial virus, metapneumovirus, influenza, parainfluenza, rhinovirus, and, more recently, Sars-CoV-2, which has been responsible for the COVID-19 pandemic. The objective of our study was to evaluate clinical data from a viral panel performed in children hospitalized with SARS or COVID-19 in the infirmary or ICU of 5 pediatric hospitals in the city of Goiânia, Goiás, Brazil. Demographic, clinical, and laboratory data were collected for analysis, and data on the outcomes underwent statistical treatment. A total of 128 patients were selected for the study, 54% of whom were male and 46% female. The viral panel included rhinovirus, COVID-19, metapneumovirus, adenovirus, and parainfluenza. Descriptive analyses of age profile showed differences in the involvement of particular viruses. The percentage of patients who required hospitalization in the ICU, infirmary, as well as individuals who were discharged after therapy or who died, were described. Our work shows that epidemiological surveillance measures are indispensable, especially if used in the continued analysis of viral panels in all pediatric patients with SARS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute respiratory infections (ARIs) are a group of diseases caused by different microorganisms. Viral etiologies, responsible for 80% of cases, affect the alveoli, bronchioles, bronchi, and interstitial space [1]. Among the viruses most frequently involved, the human respiratory syncytial virus (hRSV), human metapneumovirus (hMPV), influenza A and B, parainfluenza virus 1, 2, 3, and rhinovirus are noteworthy [2]. Recently, the new coronavirus pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, had a major impact in 2020, with several measures adopted by public health agencies in several countries aimed at controlling the spread [3, 4]. Although viral contagion is found throughout the year, the circulation of respiratory viruses in Brazil occurs predominantly in the autumn (March 20 to June 20) and winter (June 21 to September 20), thus resulting in a significant increase in prevalence during those seasons that coincides with periods when people are concentrated in closed environments with little circulation of fresh air [5].
Viral infections are likely to dysregulate the immune system, namely by inducing cytokine storms with a great capacity to cause endothelial dysfunction and the disruption of coagulation, all coincidental to changes in microvascular permeability. That severe combination may result in tissue edema and shock [6].
The viral pathogens mentioned induce infection in nearly all children in their early years, thereby resulting in high rates of morbidity, mortality, and increased health care expenditures across the world. They are also responsible for most ARIs in children, most ARIs in the community, most emergency cases, and the greatest occupancy of hospital beds and can sometimes cause sepsis [7-12].
The monitoring of patients hospitalized due to severe ARIs is paramount for epidemiological surveillance and thus public health. It also serves as an alert mechanism for potential pandemic viruses [13] and, in response, efforts to prevent further spread.
With the emergence of the COVID-19 pandemic, studies have suggested probable changes in the seasonality of ARI due to social isolation and the anticipation of vaccination for influenza. In our work, we aimed to evaluate data from a viral panel performed among children hospitalized with ARIs at the beginning of the pandemic in pediatric hospitals in the city of Goiânia, Goiás, Brazil.
Materials and methods
Ethical aspects
All of the study’s protocols for collecting and processing the samples were approved by the Ethics Committee of Hospital das Clínicas, Goiânia, under registration protocol number 33540320.7.0000.5078. The hospital units that participated in the study were the Hospital da Criança de Goiânia, Hospital Estadual de Urgências da Região Noroeste de Goiânia Governador Otávio Lage de Siqueira, Hospital Materno infantil, Hospital de Doenças Tropicais, and Neonatal Intensive Care Unit at Hospital das Clínicas of Universidade Federal de Goiás.
Inclusion criteria and data collection
The sample included all children who had acute respiratory symptoms and were admitted to the infirmary or pediatric intensive care units (ICU) in the five mentioned hospital units in Goiânia, Goiás, Brazil, in both the public and private health care systems. Also included were children with severe acute respiratory syndrome (SARS) who were seen at integrated health care centers or other inpatient services present in those units.
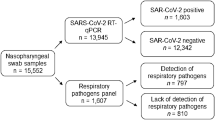
For patients to participate in the study, an authorization term or medical release form was signed by their parent or guardian. Demographic, clinical, and laboratory data were also collected for analysis, including name, age, sex, socioeconomic status, level of education, requests for laboratory exams, RT-PCR to confirm the viruses in the viral panel and SARS-CoV-2 and clinical signs, and description of clinical evolution at the end of therapy. To compose the viral panel, samples were collected using Ryon’s swab, which was inserted through the nasal cavity to the nasopharynx region to obtain the viral samples. To constitute the panel, human rhinovirus, metapneumovirus, human adenovirus, influenza A (H3N2 and H1N1) and B, human parainfluenza (1, 2, 3, and 4), and SARS-CoV-2 viruses were selected. The viral panel kit was also used for the diagnosis of human bocavirus, enterovirus, respiratory syncytial virus, and human parechovirus, although those viruses were not diagnosed in participating patients. The kit was based on PCR using TaqMan primers for the molecular detection of respiratory tract infections (i.e., specific probes for the pathogens described above), developed and supplied by Thermo Fisher Scientific™. All study data were collected from August 2020 to September 2021.
Statistical analysis
Data analyses were performed using descriptive and inferential statistics. For categorical variables in the data set, absolute and relative frequencies were calculated. For quantitative variables, measures of central tendency (i.e., mean and median) and dispersion measures (i.e., variance and standard deviation) were considered. To compare and associate categorical variables, the hypothesis Z test of the proportion for two samples and Pearson’s chi-square test were used. All variables with p ≤ 0.05 were included in the binomial logistic regression model to calculate the odds ratio. The model was validated by analyzing the diagnostics of the residuals from the distribution envelope, Akaike’s information criterion, and the Bayesian information criterion. That information was used only for model validation and is not provided herein. All hypothesis testing observed a significance level of α = 0.05.
All statistical analyses were performed in R (The R Foundation©) version 3.5.1, BioEstat® 5.3, and Stata® 16.0.
Results
Target population and viral panel
A total of 206 pediatric patients, aged from 0 to 13 years and who had symptoms of involvement of the airways, participated in the study. However, only 128 patients had confirmation by the viral panel and were selected for the case study (Supplementary Table 1). In that selected group, there were 69 males (53.90%) and 59 females (46.10%) (Fig. 1A).
As shown in Fig. 1B, when the overall percentages of viral cases presented in the viral panel were analyzed, the majority of patients tested negative for the viral panel or COVID-19 infections, for a total of 47.65% of the study’s patients. The viral groups that presented the highest number of cases were rhinovirus (18.75%), COVID-19 (16.43%), and metapneumovirus (10.15%). The other groups, including adenovirus (3.12%), co-infection with COVID-19 (3.12%), and parainfluenza (0.78%), had lower infection rates.
The indexes of male and female patients for each virus present in the viral panel were also checked (Fig. 2). Most cases were of rhinovirus, which totaled 24 cases. Among them, 13 (54.16%) were positive for males and 11 (45.84%) for females. The second-largest group was of patients affected by COVID-19, with 15 cases (71.43%) for females and six (28.57%) for males, for a total of 21 cases. The third-most frequent group was metapneumovirus, with 13 confirmed cases, nine (69.23%) of which were registered for females and four (30.77%) for males. In addition, smaller groups with adenovirus and co-infections with COVID-19 had only four cases. The parainfluenza group had only one patient, who was male. However, most cases—61, all told—were characterized as negative in the viral panel (Fig. 2).
Viral panel according to the pediatric patient classification instrument
Descriptive analyses of the age profile were performed according to the viral panel (Fig. 3). For adenovirus, there was a higher level of infection in children aged 0 to 5 years, while for COVID-19 it showed a higher rate of infection in patients up to 1 year old (44.00%). In addition, the other age groups for the COVID-19 group showed similarities (Fig. 3A). For metapneumovirus, the most-affected age groups were in individuals aged up to 1 year old (30.50%), between 1 and 2 years old (15.00%), and between 2 and 3 years old (30.50%), for a total of 76.00%. In relation to rhinovirus, the largest age groups that were infected were 1–2 years old (16.70%), 5–6 years old (20.80%), and 8–9 years old (12.50%) (Fig. 3A).
Profile of the viral panel according to age of patients and level of education. In A, it shows the relationship between the age of patients affected by SARS or COVID-19. In B, it shows the division between the Infant, Preschool and School groups according to the age of the patients. Each color represented in the image is an independent variable and each column a dependent variable. In C, analysis of the proportion of infections for each group was evaluated. AdV: Adenovirus, hMPV: human metapneumovirus, hRVs: human rhinovirus
Subsequently, all patients were divided into three groups—infants (n = 54), preschoolers (n = 28), and primary school children (n = 46)—after which comparative analyses were performed (Fig. 3B). For adenovirus, there were no significant differences between infants and preschoolers (p = 0.99). Regarding COVID-19, 56.00% of patients were infants, 24.00% were preschoolers, and 20.00% were primary school children. There were significant differences between the infant and preschool groups (p = 0.04) and between infants and primary school children (p = 0.01). However, there were no significant differences between the preschool and primary school groups (p = 0.99). For patients affected by metapneumovirus, the infant group accounted for 46.15%, while the preschool and primary school groups accounted for 15.38% and 38.46%, respectively. In that case, there were no significant differences between groups (p < 0.05). Regarding patients infected with rhinovirus, the most-affected groups were primary school children (54.00%), followed by infants (25.00%) and preschoolers (21.00%). The primary school children did not show significant differences compared with the preschool and primary school groups (p = 0.07 and p = 0.99).
However, the preschool and primary school groups did show significant differences (p = 0.03) (Fig. 3B). In addition, the association between the viral panel, infants, preschoolers, and primary school children was also verified. In those cases, no associations were found between the variables (p = 0.05), according to the Z test of proportion and the chi-square test.
The proportion of infection was analyzed following the same pattern as in the previously reported analyses. The evaluated groups were divided into infants (i.e., 0–24 months old), preschoolers (i.e., 25–60 months old), and primary school children (i.e., 61–144 months old). The variables were considered to be independent in observing the proportion of infection with COVID-19, metapneumovirus, adenovirus, and rhinovirus (Fig. 3C). Along those lines, the infant group was the most affected by COVID-19 (58.80%), rhinovirus (35.30%), and metapneumovirus (5.80%). Regarding the preschool group, there was greater involvement with viruses such as rhinovirus (35.00%), metapneumovirus (29.40%), COVID-19 (23.50%), and adenovirus (11.70%). Involvement with rhinovirus (59.10%), COVID-19 (31.80%), and metapneumovirus (9.10%) was frequent in the primary school group. Interestingly, no adenovirus infections were recorded in the primary school group (Fig. 3C and Table 1). Thus, a higher prevalence of COVID-19 was observed in the infant group, a higher homogeneity of infections in the preschool group, and a higher prevalence of rhinovirus infections in the primary school group (Fig. 3C).
As mentioned, the infant group (i.e., 0–24 months old) had the highest rates of COVID-19 infection (Table 1). However, considering the negative cases in association analysis, there were no statistically significant associations with the independent variables, OR = 2.00 (0.82–4.84; p = 0.10). In addition, a decrease in records of rhinovirus cases was observed in infant group, OR = 0.36 (0.13–0.97; p = 0.04), as shown in Table 1.
Therapy profile in hospital units
To analyze the number of patients admitted to the ICU, infirmary, or both, each category was compared based on the 128 patients in the study (Fig. 4). The most representative group in the analysis was patients admitted to the ICU, who totaled 101 individuals (74.26%), followed by individuals admitted to the infirmary, who totaled 25 (18.38%). Four patients (2.94%) were admitted to both facilities (i.e., “ICU + Infirmary”), and six individuals (4.42%) had no records (Fig. 4A).
Analysis of length of stay of patients in hospital units and percentages analysis of deaths. In A, the image depicts the number of patients admitted in ICU and infirmary. Patients who were hospitalized in both conditions were also analyzed. The number of patients who were discharged or died was also assessed. In B, the analysis of hospitalization time of affected patients during the hospitalization period was performed. In C, the clinical outcome was evaluated, involving ICU admissions or infirmary, and whether the patients was discharged or died. AdV: Adenovirus, hMPV: human Metapneumovirus e hRVs: Rhinovirus
The number of patients who were discharged or died was also analyzed. The analysis revealed that only five patients (3.91%) died, while 123 patients (96.09%) were discharged (Fig. 4A).
Subsequently, to verify the length of hospital stay, the arithmetic mean and standard deviation between the hospitalization periods of each patient were calculated (Fig. 4B and Supplementary Table 2). Next, the minimum and maximum number of days of hospitalization for each patient affected by a virus in the viral panel was calculated. Patients infected with adenovirus had an average hospital stay of 5.00 days (SD = 6.87), with a minimum of 0 days and a maximum of 15 days of hospitalization (Fig. 4B). For patients affected by COVID-19, 3.50 days (SD = 3.23) was the average length of stay (min. = 0 days, max. = 13 days) (Fig. 4B). For the metapneumovirus group, 8.58 days (SD = 9.99) was the average hospital stay for patients, with 1 day as the minimum and 33 days as the maximum time of hospitalization (Fig. 4B). For patients affected by rhinovirus, the average stay was 4.13 days (SD = 2.65), ranging from 0 to 10 days of hospitalization (Fig. 4B). Patients co-infected with COVID-19 had a mean hospital stay of 4.04 days (SD = 1.68), with a minimum of 2 days and a maximum of 7 days of hospitalization (Fig. 4B and Supplementary Table 2).
A comparative analysis of the mean number of days hospitalized due to each specific virus in the viral panel was carried out. As noted, patients affected by metapneumovirus had a longer hospital stay than ones with COVID-19 (p = 0.03) and rhinovirus (p = 0.05). When the other groups were analyzed, no significant differences were found in the length of stay (Supplementary Table 3).
Subsequently, the clinical outcome during the patients’ hospitalization period was assessed (Fig. 4C). During the analysis, the clinical outcome among the patients studied in relation to the viral panel showed similar results. Regarding the viral panel, the groups that were hospitalized and went through infirmary, ICU, and discharge had COVID-19 (2.34%) or were negative (1.56%). For patients admitted to the infirmary and after discharge, the most frequent viral types were adenovirus (1.56%), COVID-19 (3.91%), metapneumovirus (3.91%), and negative (5.50%). For patients who were admitted to the ICU and subsequently discharged, the breakdown was adenovirus (1.56%), COVID-19 (11.72%), metapneumovirus (5.47%), rhinovirus (17.97%), and negative (33.80%) (Fig. 4C). The number of deaths caused by each virus studied in the viral panel was also analyzed. Of the patients who died, two were positive for COVID-19, one for metapneumovirus, and one for rhinovirus, with a relative frequency of 8.70%, 7.69%, and 4.17%, respectively (Table 2, Figs. 4C and 5). Remarkably, the patients who died from metapneumovirus or rhinovirus and one of the patients with COVID-19 had previously been admitted to the ICU, whereas only one of the patients with SARS-CoV-2 confirmed to have died was admitted to the infirmary (Fig. 4C).
Common symptoms of patients with positive viral panel results
The symptoms presented by the patients were also investigated (Supplementary Figs. 1 and 2). Among them, the symptoms that stood out the most were fever (25.78%), measured by parents and reported during the anamnesis, measured by the medical team, or recorded in both cases. Symptoms such as tachypnea (18.75%), cough (18.75%), dyspnea (17.96%), inappetence (11.71%), vomiting (6.25%), abdominal pain (5.46%), rhinorrhea (5.46%), diarrhea (4.68%), and nasal obstruction (4,68%) were also frequent. Other symptoms such as sneezing, headache, edema, lymphadenomegaly, and conjunctivitis had a low incidence in the analyses (Supplementary Fig. 1).
We next investigated which symptoms were more frequent for each virus present in the viral panel (Supplementary Fig. 2). Regarding adenovirus, all patients had a 10.00% chance of having the following symptoms: fever, cough, inappetence, vomiting, diarrhea, tachypnea, dyspnea, rhinorrhea, nasal obstruction, and sneezing. For COVID-19, the most frequent symptoms were fever (23.56%), cough (8.82%), inappetence (8.82%), diarrhea (5.88%), abdominal pain (5.88%), vomiting (8.82%), tachypnea (8.82%), dyspnea (8.82%), and rhinorrhea (11.76%). Symptoms such as nasal obstruction (2.94%), headache (2.94%), and hoarseness (2.94%) were also observed as being less frequent. In metapneumovirus, symptoms such as cough (24.14%), fever (24.14%), dyspnea (17.24%), tachypnea (17.24%), sneezing (3.45%), nasal obstruction (3.45%), rhinorrhea (3.45%), edema (3.45%), and inappetence (3.45%) were the most evident. Patients affected by rhinovirus had a 16.66% chance of presenting symptoms such as fever, cough, sneezing, lack of appetite, dyspnea, and tachypnea (Supplementary Table 1 and Supplementary Fig. 2).
Subsequently, tests for the association between symptoms and the type of virus were performed (Table 3). Symptoms such as rhinorrhea (p = 0.03), cough (p = 0.02), and fever (p = 0.04) were associated with viruses such as COVID-19, metapneumovirus, and rhinovirus, respectively (Table 3). Other symptoms did not show significant differences with the type of virus. Thus, binomial logistic regression was used to calculate the odds ratios of symptoms caused by each virus studied (Table 4), which revealed that infected patients, in relation to uninfected patients, had a 11.4 times greater chance of having rhinorrhea, 4.76 times greater chance of having cough, and 0.12 times greater chance of having fever when affected by viruses such as COVID-19, metapneumovirus, and rhinovirus, respectively (Table 4).
Discussion
ARIs are quite common in Brazil, where they are responsible for high rates of medical appointments, hospitalization, and death, especially in childhood. The previous laboratory diagnosis of those pathogens is essential for the individual therapeutic approach and for the measures of collective controls. In patients who had a confirmed viral panel (52.00%) in our study, 53.90% were males, which aligns with most published results on the topic [14]. As for the respiratory viruses identified, the percentage of children infected with rhinovirus (19.00%), new coronavirus (16.00%) and, less frequently, parainfluenza (1.00%) was high. For rhinovirus, a higher prevalence of infections was observed in school-age pediatric patients. Similar studies have demonstrated the presence of viruses that affect the respiratory tract. However, the percentages of viral types differed from those in our study [15, 16]. Such variation can be explained by seasonal variation, the geographic location where the studies were carried out, and, primarily, by the presence of the new coronavirus, which forced changes in health habits [5, 17].
In Brazil, until the 53rd week of the epidemic (i.e., 12/27/2020 to 01/02/2021), 56,513 cases of children between the ages of < 1 to 5 years old were hospitalized with severe acute respiratory syndrome. Among those cases, 13.39% were confirmed to have COVID-19 (7,566), 1.00% (558) to have influenza, 4.00% (2442) to have other respiratory viruses, and 68.00% (38,320) to not have the etiologic agent identified, a percentage that is close to published results [18, 19]. In addition, our data showed that among the three school groups, the infant group was the most affected by SARS-CoV-2 (50.00% of the cases registered for the group).
The age group frequently affected by those viral types, except rhinovirus, was less than 1 year old, and the age group most frequently observed was 5–6 years old. In Oliveira and Soares’s study [14], 59.48% of the children who arrived in the emergency room with respiratory complaints were less than 1 year old, which our results corroborate. Those findings, according to Christ-Crain and Müller [20], can be explained by the anatomical, physiological, and immunological peculiarities of children that make them more susceptible to respiratory infections.
Among the primary symptoms associated with viral infections, fever (n = 33), tachypnea (n = 24), cough (n = 24), dyspnea (n = 23), and inappetence (n = 15) were the primary symptoms diagnosed in the study’s participants. Less frequently, hoarseness (n = 1), conjunctivitis (n = 1), and lymphadenomegaly (n = 1) were reported. Research carried out by Chen et al. [21] showed results similar to ours, thereby demonstrating that, regardless of viral type, patients have fever, cough, and dyspnea.
According to Tregoning and Schwarze [22], the lack of international definitions makes the described pathologies dubious, even irrelevant clinical diagnoses, for the treatment does not depend on those distinctions. Instead, treatment depends on the severity of the involvement of the respiratory system, the measurement of O2 saturation, blood gases and the severity of discomfort and respiratory exhaustion, increased CO2 retention, and respiratory acidosis. The authors proposed that the evolution of respiratory impairment generates acute inappetence and other additional risks, including the worsening of preexisting diseases, which can directly influence clinical management.
Anywhere in the world, patients hospitalized due to viral respiratory infections can succumb to death. As evidenced by Goka et al. [23], of 10,501 patients hospitalized with respiratory infection by viruses, 530 (5.10%) were admitted to the ICU, and 224 (2.10%) died. Patients with hRSV and hPIV1–3 infections were more likely to be admitted to an infirmary, an ICU, and progress to death. In our study, 74.26% of patients required ICU admission; however, only 3.90% of those patients died, which reflects the mentioned data.
In our study, two children died due to hMPV and hRSV. Moe et al. [24] observed that hMPV manifests itself clinically, regardless of co-detection with other viruses. In addition, the clinical manifestations and final diagnoses in children with hMPV and hRSV are quite similar. Children infected with hMPV or hRSV have variations in the clinical course of the disease. Those variations occur primarily in relation to age, because premature children and/or those with chronic diseases have an increased risk of developing severe respiratory diseases when submitted to hospitalization compared with other individuals infected with hMPV and hRSV.
It is important to note that, in Brazil until the 32nd epidemiological week of 2019, 3,514 deaths due to SARS were reported, which corresponds to 11.70% (3,514/29,978) of the total cases. Of the total reported deaths, 26.10% were due to influenza virus: 64.20% due to influenza A (H1N1), 20.50% to non-subtyped influenza A, 5.80% to influenza B, and 9.50% (87/917) to influenza A (H3N2). Regarding deaths from other respiratory viruses, 70.40% were due to hRSV [19, 25].
The absence of respiratory viruses in nearly half (48.00%) of our study’s population, could be justified by the presence of other non-viral pathogens [15], the limited amount of viral agents tested by the viral panel, and/or by the time taken to collect the biological sample, for the greatest transmissibility of the disease occurs from one day before the onset of symptoms to the seventh evolution day, and respiratory samples should preferably be collected at the beginning of the clinical picture (condition) [26].
The circulation of respiratory viruses in Brazil occurs primarily in the autumn and winter [5]. Although they occur more frequently in autumn and winter, our data from the viral panel show an increase in the frequency of individuals affected by respiratory viruses throughout the year, as shown in Table 1 (i.e., date of hospitalization). It is believed that in addition to the climatic factor, the increase occurs due to crowding and contact with infected people, usually in closed spaces with poor ventilation.
The spread of SARS-CoV-2 infections throughout Brazil urged adjustments in the health care system as it faced exceptional demand for care related to respiratory diseases. In addition to assisting patients with the new coronavirus, hospitals have also faced the traditional peak of hospitalizations for infections that affect the respiratory tract [21, 27]. To protect the population against the contamination of SARS-CoV-2, social detachment and protective measures were promoted, including improving hygiene habits and wearing a mask to protect the airways. According to the Pan American Organization of Brazil, those changes in habits reduced cases of infection in the upper respiratory tract caused by viruses by 70.00% [17]. In our research, among the children whose deaths were registered due to viral infection, two died with a confirmed diagnosis of COVID-19. According to the bulletin of 8th epidemiological week (i.e., 21–27/02/2021), 10,540 children < 1 to 5 years of age were hospitalized with SARS. Among them, 1,208 had COVID-19, and 28 died from influenza, 630 from other respiratory viruses, and 65 with COVID-19 (BRASIL, 2021). The American Academy of Pediatrics and the Children’s Hospital Association [28] reported that of the 1.4 million children diagnosed with COVID-19 as of December 2020, 154 had died. Per Koh et al. [29], children and young people have a low death rate due to COVID-19.
Zimmermann and Curtis [30] have described how children are far less likely to develop serious diseases from the COVID-19 pandemic. Although Hyde [31] claims that the statement remains true, there is therefore a perception that children are less susceptible to infection and do not promote viral transmission. However, recent evidence suggests caution, for children can be more susceptible than previously thought and can be important agents in the transmission of the disease in the community.
Detection of pediatric cases can be particularly difficult due to the high prevalence of asymptomatic infection in children [31]. In a study conducted by Waterfield et al., [32], 50.00% of infections were asymptomatic among the children of health care professionals in the United Kingdom. In South Korea, Han et al. [33] identified pediatric cases by using contact screening, in which 66.00% of children presented milder symptoms that went unnoticed and 9.00% had characteristic initial symptoms that were diagnosed. However, in our study, 48.00% of case records were negative. It is believed that those high rates of negative cases may relate to the difficulty of diagnosis. In addition, amid a pandemic, any obvious pulmonary symptom could be considered to indicate SARS, thereby leading to RT-PCR tests being performed for confirmation.
In Brazil, unlike in developed countries, the COVID-19 pandemic and other respiratory viruses have resulted in serious consequences for children’s health. There are greater risks due to some factors such as the demographic composition of the population, a greater number of children with chronic diseases, lack of access to primary health care, lack of access to centers with a quality pediatric hospital, and, primarily, social vulnerability [34]. However, if all those factors were remedied, then they could prevent or mitigate the morbidity and mortality of children affected by SARS.
Our work has demonstrated at a small scale how some viruses that cause acute respiratory syndrome were detected at the beginning of the COVID-19 pandemic. In recent years, Brazil has made a lot of improvement in the detection of respiratory viruses with more modern laboratory techniques, as well as by using an epidemiological surveillance system that analyses the behavior of the disease and how it influences social and economic aspects in the country.
With the COVID-19 pandemic, Brazil has adapted and focused its strategies on the detection of diseases related to respiratory viruses, for some of those viruses generate infection with the same symptoms as COVID-19 and lead to severe acute respiratory syndrome and sometimes to death. Viral panels performed using molecular techniques have become routine in public laboratories and robustly aided the identification and treatment of diseases. However, the primary strategy of the Brazilian government has been to carry out early vaccination campaigns for seasonal viruses, with the aim of protecting the population, especially against influenza, the viral agent that causes most of the morbidities and comorbidities. Those campaigns aim to reduce future expenditures on public health, as well as the collapse of the health system.
Conclusions
It is necessary to follow up on our epidemiological surveillance by performing viral panels in all patients with respiratory infections in the coming years. After the vaccination program against the new coronavirus, it will also be necessary to evaluate the behavior of the virus and whether it will reappear on a seasonal basis and/or with new mutations.
Data availability
Not applicable.
References
Bryce J, Boschi-Pinto C, Shibuya K, Black RE, Group WCHER (2005) WHO estimates of the causes of death in children. Lancet 365(9465):1147–1152
Cilla G, Oñate E, Perez-Yarza EG, Montes M, Vicente D, Perez-Trallero E (2008) Viruses in community-acquired pneumonia in children aged less than 3 years old: high rate of viral coinfection. J Med Virol 80(10):1843–1849
Yeoh DK, Foley DA, Minney-Smith CA, Martin AC, Mace AO, Sikazwe CT, Le H, Levy A, Blyth CC, Moore HC (2021) Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis 72(12):2199–2202
Ahmed F, Zviedrite N, Uzicanin A (2018) Effectiveness of workplace social distancing measures in reducing influenza transmission: a systematic review. BMC Public Health 18(1):1–13
Saúde Md (2020) Doença pelo coronavírus 2019: ampliação da vigilância, medidas não farmacológicas e descentralização do diagnóstico laboratorial. Bol Epidemiol 5:1–11
Steinberg B, Goldenberg N, Lee W (2012) Do viral infections mimic bacterial sepsis? The role of microvascular permeability: a review of mechanisms and methods. Antiviral Res 93(1):2–15
Moynihan KM, McGarvey T, Barlow A, Heney C, Gibbons K, Clark JE, Schlebusch S, Schlapbach LJ (2020) Testing for common respiratory viruses in children admitted to pediatric intensive care: epidemiology and outcomes. Pediatr Crit Care Med 21(6):e333–e341
Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375(9725):1545–1555
Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD (2006) Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J 25(8):680–686
Straliotto SM, Siqueira MM, Machado V, Maia TM (2004) Respiratory viruses in the pediatric intensive care unit: prevalence and clinical aspects. Mem Inst Oswaldo Cruz 99:883–887
Gupta N, Richter R, Robert S, Kong M (2018) Viral sepsis in children. Front Pediatr 6:252
Lin G-L, McGinley JP, Drysdale SB, Pollard AJ (2018) Epidemiology and immune pathogenesis of viral sepsis. Front Immunol 9:2147
Organization PAH (2011) Operational guidelines for intensified national SARI surveillance. Edited by PAHO Washington, DC
Oliveira JBdS, Soares MEdSM (2013) Perfil epidemiológico da insuficiência respiratória aguda em crianças internadas na Unidade de Terapia Intensiva de um hospital público da Paraíba. Revista InterScientia 1(3):115–126
Miyao C, Gilio A, Vieira S, Hein N, Pahl M, Betta S, Durigon E, Stewien K, Queiroz D, Botoso V (1999) Viral infections in hospitalized children affected by acute lower respiratory tract disease. Jornal de Pediatria 75(5):334–344
Ferlini R, Pinheiro FO, Andreolio C, Carvalho PRA, Piva JP (2016) Characteristics and progression of children with acute viral bronchiolitis subjected to mechanical ventilation. Revista Brasileira de Terapia Intensiva 28:55–61
Brasil O (2020) Folha informativa–COVID-19: doença causada pelo novo coronavírus
Saúde Md (2021) Boletim epidemiológico especial n° 44. Semana epidemiológica 53 (27/12/2020 a 02/1/2021)
Luz AD, Gertner S, Lessa F, Olivar M, Brasil I, Pacheco M, Barbosa CdA, Sixel C, Eto F, Murtinho R (2020) Boletim Epidemiológico vol. 13: situação epidemiológica da COVID-19 entre trabalhadores da Fiocruz: semanas epidemiológicas 10 a 44
Christ-Crain M, Müller B (2007) Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J 30(3):556–573
Chen Z-M, Fu J-F, Shu Q, Chen Y-H, Hua C-Z, Li F-B, Lin R, Tang L-F, Wang T-L, Wang W (2020) Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr 16(3):240–246
Tregoning JS, Schwarze J (2010) Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 23(1):74–98
Goka E, Vallely P, Mutton K, Klapper P (2015) Single, dual and multiple respiratory virus infections and risk of hospitalization and mortality. Epidemiol Infect 143(1):37–47
Moe N, Krokstad S, Stenseng IH, Christensen A, Skanke LH, Risnes KR, Nordbø SA, Døllner H (2017) Comparing human metapneumovirus and respiratory syncytial virus: viral co-detections, genotypes and risk factors for severe disease. PLoS ONE 12(1):e0170200
Saúde Md (2020) Boletim epidemiológico especial n° 26. Semana Epidemiol 32:7
Balasubramanian S, Rao NM, Goenka A, Roderick M, Ramanan AV (2020) Coronavirus disease 2019 (COVID-19) in children-what we know so far and what we do not. Indian Pediatr 57(5):435–442
Saúde Md Boletim epidemiológico especial n° 48. Semana epidemiológica 4 (24/01/2021 a 30/01/2021)
Pediatrics AAo the Children’s Hospital Association. Children and COVID-19: state data report: a joint report from the American Academy of Pediatrics and the Children’s Hospital Association. Version 12/24/20. https://downloads.aap.org. Accessed December 31, 2020
Koh HK, Geller AC, VanderWeele TJ (2021) Deaths from COVID-19. JAMA 325(2):133–134
Zimmermann P, Curtis N (2020) COVID-19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr Infect Dis J 39(6):469
Hyde Z (2020) COVID-19, children and schools: overlooked and at risk. Med J Aust 213(10):444-446.e441
Waterfield T, Watson C, Moore R, Ferris K, Tonry C, Watt A, McGinn C, Foster S, Evans J, Lyttle MD (2021) Seroprevalence of SARS-CoV-2 antibodies in children: a prospective multicentre cohort study. Arch Dis Child 106(7):680–686
Han MS, Choi EH, Chang SH, Jin B-L, Lee EJ, Kim BN, Kim MK, Doo K, Seo J-H, Kim Y-J (2021) Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic of Korea. JAMA Pediatr 175(1):73–80
Ariyo E, Amurtiya M, Lydia OY, Oludare A, Ololade O, Taiwo AP, Olukemi LA, Ogunniyi D (2022) Socio-demographic determinants of children home learning experiences during COVID 19 school closure. Int J Educ Res Open 3:100111
Funding
The research, conducted at the Universidade Federal de Goiás, was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior [Grant Nos. 0678/2020/88881.504906/2020–01].
Author information
Authors and Affiliations
Contributions
All authors contributed to conception and design of the study. The study was conceptualized by ALEM, LCC, IJW, and MAGA. Data were collected by ALEM, KMA, MOC, VCR, PPS, SBSM, and FAOP. The material for comparative and statistical analysis was prepared by ALEM, LPA, MOS, and PANS. The first draft of the manuscript was written by ALEM, PANS, and CRMI, while MOS, LCC, and MAGA revised and edited subsequent versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The research was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of Hospital das Clínicas—GO (HC-UFG) (33540320.7.0000.5078) for studies involving humans.
Consent to participate
No informed consent was required from participants.
Consent for publication
All authors have consented to publication of the manuscript.
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moreira, A.L.E., da Silva, P.A.N., Assunção, L.d. et al. Profile analysis of emerging respiratory virus in children. Eur J Clin Microbiol Infect Dis 42, 873–882 (2023). https://doi.org/10.1007/s10096-023-04615-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04615-8