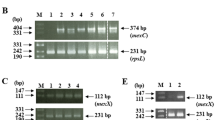
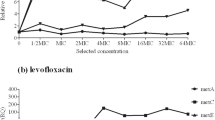
Abstract
Antimicrobial resistance is a major public-health concern. We evaluate chlorhexidine role in selection of resistant Pseudomonas aeruginosa mutants and their antibiotic cross-resistance. Mutation frequency and mutation rate after short-term exposure to sub-inhibitory concentrations of chlorhexidine were compared to those after spontaneous chlorhexidine-exposure, in P. aeruginosa PAO1 strain. Chlorhexidine-resistant mutants were generated, either by serial passage in increasing chlorhexidine concentrations or by single exposure to lethal chlorhexidine concentration. The generated mutants were tested for cross-resistance to different antibiotics, by determination of minimum inhibitory concentrations (MIC). The accompanied phenotypic changes in membrane permeability, outer membrane proteins (OMP), and efflux function were evaluated. The effect of exposure to chlorhexidine on MexAB-OprM, MexEF-oprN, and MexXY efflux pumps expression was investigated. No significant change was recorded between the mutation frequencies and mutation rates after short-term exposure to sub-inhibitory concentrations of chlorhexidine and after spontaneous chlorhexidine-exposure, in P. aeruginosa PAO1 strain. Twelve stable mutants, with ≥ eight-fold increase in chlorhexidine MIC, were generated. Several mutants showed increase in the MIC of colistin, cefepime, ceftazidime, meropenem, ciprofloxacin, and amikacin; seven mutants expressed meropenem cross-resistance. This was accompanied by decreased outer membrane permeability and changes in OMP. Using efflux pump inhibitor, chlorhexidine resistance was reverted in most isolates. Exposure to sub-inhibitory concentration of chlorhexidine induced the expression of MexXY efflux pump. Some resistant mutants had overexpressed MexXY efflux pump. Chlorhexidine can select P. aeruginosa strains with antibiotic cross-resistance. This necessitates implementing special protocols for chlorhexidine use and re-evaluation of its benefit versus risk in personal-care products.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Centers for Disease Control and Prevention. 2019 AR threats report [Internet]. 2019; https://www.cdc.gov/drugresistance/biggest-threats.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fdrugresistance%2Fbiggest_threats.html
Willyard C (2017) The drug-resistant bacteria that pose the greatest health threats. Nat News 543:15
Chuanchuen R, Beinlich K, Hoang TT, Becher A, Karkhoff-Schweizer RR, Schweizer HP (2001) Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob Agents Chemother. 45:428–432. https://www.ncbi.nlm.nih.gov/pubmed/11158736: https://doi.org/10.1128/AAC.45.2.428-432.2001
Morita Y, Murata T, Mima T, Shiota S, Kuroda T, Mizushima T et al (2003) Induction of mexCD-oprJ operon for a multidrug efflux pump by disinfectants in wild-type Pseudomonas aeruginosa PAO1. J Antimicrob Chemother 51:991–994. https://doi.org/10.1093/jac/dkg173
Slipski CJ, Zhanel GG, Bay DC (2018) Biocide selective TolC-independent efflux pumps in Enterobacteriaceae. J Membr Biol 251:15–33. https://doi.org/10.1007/s00232-017-9992-8
FDA issues final rule on safety and effectiveness of antibacterial soaps | FDA. FDA (2016) [cited 2019 24]; https://www.fda.gov/news-events/press-announcements/fda-issues-final-rule-safety-and-effectiveness-antibacterial-soaps. Accessed 29 May 2017
Horner C, Mawer D, Wilcox M (2012) Reduced susceptibility to chlorhexidine in staphylococci: is it increasing and does it matter? J Antimicrob Chemother 67:2547–2559. https://doi.org/10.1093/jac/dks284
Stickler DJ, Thomas B (1980) Antiseptic and antibiotic resistance in Gram-negative bacteria causing urinary tract infection. J. Clin Pathol. 33:288–96. https://www.ncbi.nlm.nih.gov/pubmed/6769972https://doi.org/10.1136/jcp.33.3.288
Stein C, Vincze S, Kipp F, Makarewicz O, Al Dahouk S, Pletz MW (2019) Carbapenem-resistant Klebsiella pneumoniae with low chlorhexidine susceptibility. Lancet Infect Dis 19:932–933. https://doi.org/10.1016/S1473-3099(19)30427-X
Wand ME, Bock LJ, Bonney LC, Sutton JM (2017) Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob Agents Chemother 61:1–31. https://doi.org/10.1128/AAC.01162-16
Lister PD, Wolter DJ, Hanson ND (2009) Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 22:582–610. https://doi.org/10.1128/CMR.00040-09
World Health Organization (2017) WHO publishes list of bacteria for which new antibiotics are urgently needed. https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. Accessed March 2018
Ostroff RM, Wretlind B, Vasil ML (1989) Mutations in the hemolytic-phospholipase C operon result in decreased virulence of Pseudomonas aeruginosa PAO1 grown under phosphate-limiting conditions. Infect Immun 57:1369–1373
Wayne, PA (2015) Clinical and Laboratory Standards Institute. M07-A10: methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard—Tenth Edition
Tsuchiya K, Cao Y-Y, Kurokawa M, Ashino K, Yomo T, Ying B-W (2018) A decay effect of the growth rate associated with genome reduction in Escherichia coli. BMC Microbiol 18:101. https://doi.org/10.1186/s12866-018-1242-410.1186/s12866-018-1242-4
Birosova L, Mikulasova M (2009) Development of triclosan and antibiotic resistance in Salmonella enterica serovar Typhimurium. J Med Microbiol. 58:436–41. http://jmm.microbiologyresearch.org/content/journal/jmm/https://doi.org/10.1099/jmm.0.003657-0
Rosche WA, Foster PL (2000) Determining mutation rates in bacterial populations. Methods 20:4–17. https://doi.org/10.1006/meth.1999.0901
Lanini S, D’Arezzo S, Puro V, Martini L, Imperi F, Piselli P, et al (2011) Molecular epidemiology of a Pseudomonas aeruginosa hospital outbreak driven by a contaminated disinfectant-soap dispenser. PLoS One. 6. https://doi.org/10.1371/journal.pone.0017064
Clinical and Laboratory Standards Institute (2017) Performance standards for antimicrobial susceptibility testing; Twenty-Seventh Informational Supplement. CLSI Document M100-S27. www.clsi.org
Lamers RP, Cavallari JF, Burrows LL (2013) The efflux inhibitor phenylalanine-arginine beta-naphthylamide (PAβN) permeabilizes the outer membrane of gram-negative bacteria. PLoS ONE 8:1–7. https://doi.org/10.1371/journal.pone.0060666
Murphy TF, Loeb MR (1989) Isolation of the outer membrane of Branhamella catarrhalis. Microb Pathog 6:159–174. https://doi.org/10.1016/0882-4010(89)90066-1
Mohamed SA (2015) A study of the role of interaction between Moraxella catarrhalis surface antigen and specific host antibody response on the occurance of otitis media. Master thesis, Cairo University
Bradford MM (1976) A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Kristiansen JE, Thomsen VF, Martins A, Viveiros M, Amaral L (2010) Non-antibiotics reverse resistance of bacteria to antibiotics. In Vivo (Brooklyn) 24:751–754
Thomas L, Maillard J, Lambert RJW, Russell AD (2000) Development of resistance to chlorhexidine diacetate in Pseudomonas aeruginosa and the effect of a ‘ residual ’ concentration. 297–303. https://doi.org/10.1053/jhin.2000.0851
Kampf G (2019) Adaptive bacterial response to low level chlorhexidine exposure and its implications for hand hygiene. Microb. cell (Graz, Austria) 6:307–320. https://doi.org/10.15698/mic2019.07.683
Hashemi MM, Holden BS, Coburn J, Taylor MF, Weber S, Hilton B, et al (2019) Proteomic analysis of resistance of Gram-negative bacteria to chlorhexidine and impacts on susceptibility to colistin , antimicrobial peptides, and ceragenins. 10:1–13. https://doi.org/10.3389/fmicb.2019.00210
Forbes S, Dobson CB, Humphreys GJ, McBain AJ (2014) Transient and sustained bacterial adaptation following repeated sublethal exposure to microbicides and a novel human antimicrobial peptide. Antimicrob Agents Chemother. 58:5809–17. https://www.ncbi.nlm.nih.gov/pubmed/25049246https://doi.org/10.1128/AAC.03364-14
Tattawasart U, Maillard JY, Furr JR, Russell AD (1999) Development of resistance to chlorhexidine diacetate and cetylpyridinium chloride in Pseudomonas stutzeri and changes in antibiotic susceptibility. J Hosp Infect 42:219–229. https://doi.org/10.1053/jhin.1999.0591
Bock LJ, Wand ME, Sutton JM (2016) Varying activity of chlorhexidine-based disinfectants against Klebsiella pneumoniae clinical isolates and adapted strains. J Hosp Infect 93:42–48. https://doi.org/10.1016/j.jhin.2015.12.019
Bhardwaj P, Hans A, Ruikar K, Guan Z, Palmer KL (2017) Reduced chlorhexidine and daptomycin susceptibility in vancomycin-resistant Enterococcus faecium after serial chlorhexidine exposure. Antimicrob. Agents Chemother 62: e01235–17. https://doi.org/10.1128/AAC.01235-17
Doi Y (2019) Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin Infect Dis 69:S565-S575. https://doi.org/10.1093/cid/ciz830
Kampf G (2018) Biocidal agents used for disinfection can enhance antibiotic resistance in gram-negative species. Antibiotics 7:110. https://doi.org/10.3390/antibiotics7040110
Kramer A, Behrens-Baumann W (2002) Antiseptic prophylaxis and therapy in ocular infections: principles, clinical practice and infection control. Karger Medical and Scientific Publishers
Delcour AH (2009) Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta 1794:808–816. https://doi.org/10.1016/j.bbapap.2008.11.005
Denyer SP, Maillard J-Y (2002) Cellular impermeability and uptake of biocides and antibiotics in Gram-negative bacteria. J Appl Microbiol 92(Suppl):35S-45S
Brozel VS, Cloete TE (1994) Resistance of Pseudomonas aeruginosa to isothiazolone. J Appl Bacteriol 76:576–582
Yamano Y, Nishikawa T, Komatsu Y (1990) Outer membrane proteins responsible for the penetration of beta-lactams and quinolones in Pseudomonas aeruginosa. J Antimicrob Chemother 26:175–184. https://doi.org/10.1093/jac/26.2.175
Alonso A, Campanario E, Martínez JL (1999) Emergence of multidrug-resistant mutants is increased under antibiotic selective pressure in Pseudomonas aeruginosa. Microbiology 145:2857–2862. https://doi.org/10.1099/00221287-145-10-2857
Tabata A, Nagamune H, Maeda T, Murakami K, Miyake Y, Kourai H (2003) Correlation between resistance of Pseudomonas aeruginosa to quaternary ammonium compounds and expression of outer membrane protein OprR 1. Antimicrob. Agents Chemother. 47:2093–9. isi:000183786700005 https://doi.org/10.1128/AAC.47.7.2093
Morita Y, Tomida J, Kawamura Y (2014) Responses of Pseudomonas aeruginosa to antimicrobials. Front Microbiol 4:422. https://doi.org/10.3389/fmicb.2013.00422
Young ML, Bains M, Bell A, Hancock RE (1992) Role of Pseudomonas aeruginosa outer membrane protein OprH in polymyxin and gentamicin resistance: isolation of an OprH-deficient mutant by gene replacement techniques. Antimicrob Agents Chemother 36:2566–2568. https://doi.org/10.1128/aac.36.11.2566
Gilbert P, Moore LE (2005) Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol 99:703–715. https://doi.org/10.1111/j.1365-2672.2005.02664.x
Poole K (2005) Efflux-mediated antimicrobial resistance. J Antimicrob Chemother 56:20–51. https://doi.org/10.1093/jac/dki171
Mombeshora M, Mukanganyama S (2017) Development of an accumulation assay and evaluation of the effects of efflux pump inhibitors on the retention of chlorhexidine digluconate in Pseudomonas aeruginosa and Staphylococcus aureus. BMC Res Notes 10:1–9. https://doi.org/10.1186/s13104-017-2637-2
Amsalu A, Sapula SA, De Barros Lopes M, Hart BJ, Nguyen AH, Drigo B, et al (2020) Efflux pump-driven antibiotic and biocide cross-resistance in Pseudomonas aeruginosa isolated from different ecological niches: a case study in the development of multidrug resistance in environmental hotspots. Microorganisms. 24;8:1647. https://pubmed.ncbi.nlm.nih.gov/33114277https://doi.org/10.3390/microorganisms8111647
Aeschlimann JR (2003) The role of multidrug efflux pumps in the antibiotic resistance of Pseudomonas aeruginosa and other gram-negative bacteria. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 23:916–24
Hocquet D, Muller A, Blanc K, Plésiat P, Talon D, Monnet DL et al (2008) Relationship between antibiotic use and incidence of MexXY-OprM overproducers among clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:1173–1175. https://doi.org/10.1128/AAC.01212-07
Li X-Z, Plésiat P (2016) Antimicrobial drug efflux pumps in Pseudomonas aeruginosa. In: Li X-Z, Elkins CA, Zgurskaya HI, editors. Efflux-mediated antimicrobial resistance in bacteria. 1st ed. Cham: Springer International Publishing. pp. 359–400. https://doi.org/10.1007/978-3-319-39658-3_14
Acknowledgements
The authors would like to thank Dr. Ahmed Sherif Attia (Cairo University) for providing P. aeruginosa PAO1 strain, Dr. Mohamed Abdel Halim Ramadan (Cairo University) for providing chlorhexidine hydrochloride, and Dr. Shahira Abdel Salam (Cairo University) for her technical assistance.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.S.Y., O.E., and M.T.K.; methodology, M.A.T; formal analysis, M.A.T., A.S.Y., and M.T.K.; writing—original draft preparation, M.A.T.; writing—review and editing, A.S.Y. and M.T.K.; supervision, A.S.Y. and M.T.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the ethics committee of Faculty of Pharmacy, Cairo University (approval number: MI 1617).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tag ElDein, M.A., Yassin, A.S., El-Tayeb, O. et al. Chlorhexidine leads to the evolution of antibiotic-resistant Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis 40, 2349–2361 (2021). https://doi.org/10.1007/s10096-021-04292-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-021-04292-5