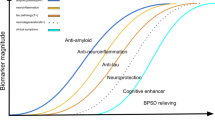
Abstract
Objectives
Masitinib, originally developed as a tyrosine kinase inhibitor for cancer treatment, has shown potential neuroprotective effects in various neurological disorders by modulating key pathways implicated in neurodegeneration. This scoping review aimed to summarize the current evidence of masitinib’s neuroprotective activities from preclinical to clinical studies.
Methods
This scoping review was conducted following the guidelines described by Arksey and O’Malley and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The inclusion criteria covered all original studies reporting on the neuroprotective effects of masitinib, including clinical studies, animal studies, and in vitro studies.
Results
A total of 16 studies met the inclusion criteria and were included in the review. These comprised five randomized controlled trials (RCTs), one post-hoc analysis study, one case report, and nine animal studies. The RCTs focused on Alzheimer’s disease (two studies), multiple sclerosis (two studies), and amyotrophic lateral sclerosis (one study). Across all included studies, masitinib consistently demonstrated neuroprotective properties. However, the majority of RCTs reported concerns regarding the safety profile of masitinib. Preclinical studies revealed the neuroprotective mechanisms of masitinib, which include inhibition of certain kinases interfering with cell proliferation and survival, reduction of neuroinflammation, and exhibition of antioxidant activity.
Conclusion
The current evidence suggests a promising therapeutic benefit of masitinib in neurodegenerative diseases. However, further research is necessary to validate and expand upon these findings, particularly regarding the precise mechanisms through which masitinib exerts its therapeutic effects. Future studies should also focus on addressing the safety concerns associated with masitinib use.
Similar content being viewed by others
Introduction
Neurological and neurodegenerative diseases include a broad spectrum of disorders, which affect the central and peripheral nervous systems. They are often associated with functional impairments and diminished quality of life [1]. The prevalence of multiple neurological diseases is increasing [2, 3]. For instance, it is expected that the worldwide prevalence of amyotrophic lateral sclerosis (ALS) will reach 376,674 patients in 2040 with an estimated 69% growth from 222,801 patients in 2015 [2]. Hence, appropriate management strategies are essential to reduce the morbidity and mortality associated with these debilitating disorders. The management of these disorders depends on supportive care, rehabilitation, lifestyle modifications, and pharmacological interventions, which aim to alleviate symptoms, slow disease progression, and consequently minimize disability and improve overall functioning and well-being [4]. Despite the recognized importance of appropriate management strategies, the management of neurological and neurodegenerative diseases encounters various challenges, such as high healthcare costs and limited treatment options for some complex diseases [5].
The exact pathophysiology of neurodegenerative disorders is not fully understood; however, previous research suggested that chronic inflammation largely contributed to the development and progression of these diseases [6]. Given that inflammation of the central nervous system is tightly regulated by astrocytes and microglia, it is hypothesized that manipulation of these cells may provide a possible therapeutic option against these diseases [7]. Therefore, researchers have explored the potential therapeutic benefits of masitinib, an oral selective tyrosine kinase inhibitor that was originally developed as an anticancer drug, in various neurodegenerative disorders, such as Alzheimer’s disease (AD), ALS, and multiple sclerosis (MS) [8,9,10]. Indeed, preclinical and clinical studies have shown promising results regarding the efficacy of masitinib in neurodegenerative disorders via inhibition of microglia, astrocytes, and mast cell activity in both central and peripheral nervous systems [9,10,11]. However, randomized controlled trials (RCTs) investigating the efficacy of masitinib in these disorders are still limited, and further well-designed RCTs are warranted to validate the current evidence. In addition, it was also hypothesized that masitinib may improve the prognosis of ischemic stroke because mast cells may participate in the development of ischemic stroke. Therefore, animal studies have been conducted to test this hypothesis [12].
Interestingly, these studies have shown promising results, suggesting that masitinib may be an effective adjunct agent in stroke management. Importantly, the potential benefits, safety profile, and different mechanisms of action of masitinib in all aforementioned neurological diseases have not been summarized in previous reviews. Therefore, we conducted this scoping review to summarize the available evidence regarding the role of masitinib as a neuroprotective agent.
Methods
Protocol and registration
This scoping review was conducted following the guidelines described by Arksey and O’Malley and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13, 14]. Also, the final results are reported according to PRISMA and PRISMA-Scoping Review guidelines [13, 15]. This review was registered in the PROSPERO international prospective register of systematic reviews (registration number CRD42023457214).
Search strategy
PubMed, Scopus, Web of Science, and Cochrane CENTRAL databases were searched up to August 1, 2023. To ensure a comprehensive search, we focused on using masitinib-related terms exclusively and did not include any neurological terms (“Masitinib” OR “AB1010” OR “Kinavet” OR “Masivet”). No filters or language restrictions were applied. The references of the included studies were also screened to ensure all relevant articles were covered.
Inclusion criteria
The objective of this scoping review was to summarize the current evidence of the neuroprotective activities of masitinib from preclinical to clinical studies. Thus, our review included all original studies reporting on the neuroprotective effects of masitinib. There were no restrictions placed on the studied disease, population, or outcomes. The inclusion criteria covered (a) RCTs, observational studies, case reports, and case series; (b) animal studies; and (c) in vitro studies. Reviews, editorials, and studies on non-neurological diseases were excluded.
Study selection and data extraction
Without removing duplicates, two reviewers independently screened the titles and abstracts of the citations [16]. Then, a third reviewer retrieved and screened the full text of the identified studies to make the final decision. After identifying the included studies, two authors extracted the data using an online data extraction form. For RCTs, the extracted data included information such as country, population, sample size, outcomes, and main findings. For animal studies, the extracted data encompassed the animal model, sample size, age, sex, outcomes, and main findings. Any discrepancies in the screening process or data extraction were resolved through discussion with a third author.
Quality assessment
We followed the Cochrane risk-of-bias tool for randomized trials (RoB 2.0) tool to assess the quality of the included RCTs [17]. This tool evaluates the risk of bias in five domains, including bias arising from the randomization process, deviations from intended interventions, missing outcome data, measurement of outcomes, and selection of the reported result. The trial was considered to be at high risk if at least one domain was rated as high risk, and low risk if all domains were judged as low risk. For animal studies, the CAMARADES checklist was used to assess their quality [18]. There was one case report included and was assessed using the Joanna Briggs Institute tool [19].
Analysis
Given the heterogeneity in the included studies in terms of populations and outcomes, we conducted a qualitative analysis of the data following the recommended methodology for qualitative reviews outlined in the Cochrane Handbook [20]. We categorized the manuscript into two groups: (i) preclinical and (ii) clinical. For the preclinical studies (animal studies), we tabled the following information: (1) animal model utilized (e.g., mouse, pig); (2) sample size; (3) age, sex, and weight of the animals; (4) outcome measures assessed; (5) main findings; and (6) study quality evaluation. Regarding the clinical studies, we gathered the following details: (1) study design (e.g., RCT, case study); (2) characteristics of the study population (i.e., age and disease condition); (3) outcome measures examined; (4) effects of masitinib on the outcome measures; and (5) evaluation of study quality using the aforementioned criteria.
Results
Characteristics of the included studies
Our search resulted in a total of 1261 citations. After screening the titles and abstracts, 50 records were identified and assessed for eligibility. Of which, 12 were protocols, 12 were duplicates, six were conference abstracts, three were reviews, and one article was an erratum on an included article. Finally, a total of 16 studies met the inclusion criteria and were included in our review. These consisted of five RCTs [8,9,10, 21, 22], one post-hoc analysis study [23], one case report of a patient included in an RCT [24], and nine animal studies [8, 11, 12, 25,26,27,28,29,30]. Figure 1 summarizes the selection process of the included studies. The quality of the included RCTs was generally high, as summarized in Table 1. Table 2 summarizes the quality assessment of the included animal studies, which were of moderate quality. The included case report was of good quality based on the Joanna Briggs Institute tool [24].
Evidence from the preclinical studies
Our comprehensive review identified nine preclinical studies with over 350 animals. Five studies were conducted on SOD1G93A mutant rats modeling ALS, while two studies were on C57BL/6 mice (Table 3). In their study on Wistar rats with post-ischemic stroke, Kocic et al. investigated the effect of masitinib (25 or 100 mg/kg twice daily) on reducing the infarct size and the neurological deficit after the stroke [12]. They found that masitinib alone significantly reduced the infarct size compared with the control group, and masitinib combined with tissue-type plasminogen activator was superior to tissue-type plasminogen activator alone. Masitinib also reduced the neurological symptoms compared to the control group. A similar study by Qian et al. investigated the effects of masitinib on the mechanoreception of sensory neurons in C57BL/6 mice of tourniquet-induced hind paw ischemia–reperfusion [25]. Masitinib mitigated nerve damage and improved hind paw mechanoreception to mechanical stimulation. Trias et al. investigated masitinib in a SOD1G93A mutant rats model of ALS to explore its therapeutic effects and its effects on isolated cultured aberrant glial cells [26]. They found that administration of masitinib decreased aberrant glial cells, improved motor neuron pathology, and prolonged post-paralysis survival. Similar findings were found in other studies, showing improved reinnervation and reduced regressive changes of Schwann cells [27, 30]. Another study was on a mice model of AD by Li et al., investigating the effects of masitinib on cognitive function, neuroinflammation, brain amyloidosis, and synaptic integrity [11]. Masitinib reduced the synaptic integrity and the cognitive anomalies; however, they observed no benefits regarding inflammatory mediators and microglial densities. Besides their RCTs on patients with MS, Vermersch et al. also explored the effects of masitinib using a myelin oligodendrocyte glycoprotein murine model, and found a significant reduction in disease, as assessed by the mean clinical score [8].
Evidence from the clinical studies
The included clinical studies consisted of five RCTs, along with one post hoc analysis of an RCT and one case report involving a patient from the same RCT. As demonstrated in Table 4, two RCTs were on AD [10, 21], two on MS [8, 22], and one on ALS [9]. The post-hoc analysis study was based on the RCT on ALS, and the case report was also about an ALS patient [23, 24]. In their RCT, Vermersch et al. investigated two doses of masitinib (4.5 and 6.0 mg/kg/day) in patients with primary progressive MS or nonactive secondary progressive MS [22]. They found that masitinib (4.5 mg/kg/day) showed significant benefit over placebo according to the primary endpoint. Also, in the other RCT on MS, masitinib (6.0 mg/kg/day) appeared to have a positive effect on MS-related impairment compared with patients receiving placebo [8]. A phase 2 RCT by Piette et al. investigated masitinib (3.0 or 6.0 mg/kg/day) in patients with AD and found that cognitive decline in the masitinib group was significantly lower than the placebo group [21]. Also, the placebo treatment arm showed a worsening mean regarding the AD functional scales. Dubois et al. confirmed these results in their phase three RCT, as they found significant benefits of masitinib over placebo in terms of all investigated outcomes [10].
Regarding ALS, a study conducted by Mora et al. examined the effects of masitinib (4.5 or 3.0 mg/kg/day) in ALS patients [9]. The results showed that masitinib significantly slowed down the decline in ALS functional status compared to the placebo group, with a 27% reduction in the rate of functional decline. However, it was noted that the masitinib groups experienced a higher incidence of severe adverse effects. Building upon this RCT, a subsequent long-term survival analysis was carried out to investigate the actual effects of masitinib [23]. The analysis revealed a significant survival benefit of 25 months and a 47% reduced risk of death for patients receiving a dosage of 4.5 mg/kg/day masitinib compared to the placebo group. Additionally, Salvado et al. reported a case of autoimmune-like hepatitis potentially associated with masitinib treatment in one patient from this RCT [24].
Discussion
Key findings
The objective of this scoping review was to provide an overview of the existing evidence on the neuroprotective effects of masitinib. Our investigation yielded a total of 14 studies that examined the neuroprotective effects of masitinib, consisting of five RCTs and nine animal studies. Furthermore, we included one post hoc analysis study and one case report, both derived from an included RCT. The collective findings from these studies consistently demonstrated the neuroprotective properties of masitinib. However, the majority of RCTs reported a concerning safety profile for masitinib.
Interpretation
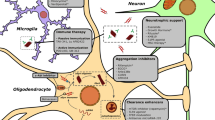
Neuroprotection refers to the preservation of neuronal structure and function, aiming to prevent or slow down the progression of neurodegenerative disorders [31]. Various mechanisms have been proposed for neuroprotection, including anti-inflammatory effects, antioxidant properties, modulation of cell survival pathways, and attenuation of excitotoxicity [7, 32]. Masitinib, a tyrosine kinase inhibitor originally developed as an anticancer agent [33], selectively targets specific kinases involved in cell signaling pathways associated with inflammation, cell survival, and tissue remodeling [34]. Masitinib’s first anticancer therapy was approved in canine mast cell tumors and showed promising results [35, 36]. Similarly in humans, masitinib showed significant survival benefit in advanced gastrointestinal stromal tumor and pancreatic cancer [37, 38]. Beyond its oncology applications, masitinib has shown potential benefits in various neurological disorders due to its ability to modulate key pathways implicated in neurodegeneration [11, 26]. Preclinical studies included in our review have demonstrated the neuroprotective mechanisms of masitinib. Firstly, masitinib inhibits certain kinases, including c-Kit, PDGFR, and Lyn, interfering with signaling pathways involved in cell proliferation, survival, and inflammation [34]. Secondly, it reduces neuroinflammation, as demonstrated by Trias et al., who found a reduction in neuroinflammation, microgliosis, and aberrant glial cells associated with masitinib use [26]. Qian et al. also reported alleviated allodynia and decreased inflammatory cytokines in the masitinib-treated group [25]. Thirdly, masitinib exhibits antioxidant activity, as Qian et al. observed a reduction in reactive oxygen species, leading to improved mechanoreception and alleviation of sensory nerve damage [25]. Figure 2 summarizes the potential neuroprotective mechanisms of masitinib.
Although the mechanisms of neurodegeneration are not fully understood, research has confirmed that immunity and inflammation are involved in the pathophysiology of several neurodegenerative diseases, such as ALS, MS, and AD [39,40,41,42]. Various immunological cell types, such as cytokines, are expressed and actively present in the brain during neurodegeneration [43]. Cytokines share in repair processes in the central nervous system by facilitating the pathogen clearance and reducing tissue damage [44]. Other cells, such as microglia and astrocytes, are also involved in the immune defense, regulating tissue homeostasis and preserving the brain structure and function [45]. However, it is still not clear whether these immunological cells are beneficial or detrimental in pathological neurological conditions. For example, over expression of cytokines may lead to apoptosis and severe inflammation [46]. Chronic activation of microglia can cause neuronal damage by releasing cytotoxic molecules [47]. Mast cells also exert significant effects on their microenvironment and neighboring cells, including astrocytes, microglia, and neurons, which are implicated in neuroinflammation and neurodegeneration [48]. The involvement of mast cells, cytokines, microglia, and astrocytes in the pathogenesis of neurodegeneration may provide insights into the potential effects of masitinib as a neuroprotective agent. Research has confirmed that masitinib can modify neuroinflammation by reducing mast cells, microgliosis, aberrant glial cells, and inflammatory cytokines [11, 26, 27, 29]. Also, the antioxidant activity of masitinib could be involved in this neuroinflammatory regulation, as oxidative stress has been linked to the progress of several neurodegenerative disorders [49].
In clinical studies included in our review, masitinib has shown promising results in terms of cognitive decline in AD, functional status in ALS, and impairment related to MS [8,9,10]. In ALS, masitinib targets microglial cells, mast cells, and macrophage infiltration, thereby attenuating neuroinflammatory processes [27, 28]. Also, reducing the reactivity of Schwann cells was addressed as a potential mechanism of masitinib in ALS, contributing to the preservation of neuronal function and slowing down the disease progression [29]. In MS and AD, modulating mast cells’ activity could improve the disruption of the blood–brain barrier and decreases the infiltration of immune cells into the central nervous system, thereby reducing inflammation and preserving neurological function [50]. Overall, masitinib’s mechanism of action in ALS, MS, and AD mostly involves targeting key components of neuroinflammation. Future research is required to enhance our understanding of masitinib’s specific mechanisms in neurodegenerative diseases. Also, future and ongoing RCTs, such as NCT03127267 and NCT05441488, will provide valuable insights and confirm the therapeutic benefits of masitinib.
Strengths and limitations
This review represents the first comprehensive evaluation of the neuroprotective effects of masitinib. We employed a thorough search strategy and followed established guidelines, ensuring a comprehensive assessment of the available evidence. The review included both preclinical studies and clinical trials, providing a broader perspective on the neuroprotective effects of masitinib. The inclusion of different study types strengthens the overall evidence base. The included studies underwent a methodological quality assessment using appropriate tools, such as RoB 2 for RCTs and the CAMARADES checklist for animal studies. However, our scoping review identified a relatively small number of studies that met the inclusion criteria. This limited pool of evidence may restrict the generalizability of the findings and highlights the need for additional research on the topic.
Clinical implications and recommendations
Masitinib is a promising and practical treatment for a wide range of neurodegenerative disorders. Multiple pharmacological targets, such as modulating the A and Tau protein signaling cascade and preventing synaptic damage, make it a potential treatment for Alzheimer’s disease-related dementia [51]. Clinical studies have shown that masitinib can slow cognitive decline in patients with mild to moderate Alzheimer’s disease [10]. Furthermore, it may be used as a treatment for progressive forms of multiple sclerosis, since it acts on growth and activation pathways to hinder mast cell survival, migration, cytokine generation, and degranulation [22]. As it targets macrophages, mast cells, and microglia cells, masitinib may be useful in treating ALS because it highlights the disease’s neuroinflammatory activity [9]. Masitinib inhibits the production of inflammatory cytokines, lessens inflammation indirectly, and triggers neuroprotection [52]. While masitinib shows promise in the treatment of various neurodegenerative disorders, further investigation is necessary to address potential adverse effects and optimize its therapeutic use. Continued research and clinical trials will help refine its application and ensure its safe and effective utilization in clinical practice.
Conclusion
Masitinib shows promising potential as a neuroprotective agent in various neurodegenerative diseases. The available evidence, including preclinical and clinical studies, suggests that masitinib exerts neuroprotective effects through its modulation of key signaling pathways implicated in cell proliferation, survival, neuroinflammation, and antioxidant activity. However, concerns regarding the safety profile of masitinib have been raised. Further research is needed to confirm and explore the therapeutic benefits of masitinib in neurodegenerative diseases. Future studies should focus on addressing the safety concerns associated with masitinib use. Additionally, investigations into optimal dose and potential combination therapies may help maximize the efficacy of masitinib as a neuroprotective agent.
Data availability
Not applicable.
References
Tang Y, Liang X, Han L et al (2020) Cognitive function and quality of life in Parkinson’s disease: a cross-sectional study. J Park Dis 10:1209–1216. https://doi.org/10.3233/JPD-202097
Arthur KC, Calvo A, Price TR et al (2016) Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat Commun 7:1–6. https://doi.org/10.1038/ncomms12408
Heitmann RC (2012) Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med 2:a006239. https://doi.org/10.1101/cshperspect.a006239
Clare L, Teale JC, Toms G et al (2019) Cognitive rehabilitation, self-management, psychotherapeutic and caregiver support interventions in progressive neurodegenerative conditions: a scoping review. NeuroRehabil 43:443–471. https://doi.org/10.3233/NRE-172353
Hardiman O, Al-Chalabi A, Chio A et al (2017) Amyotrophic lateral sclerosis. Nat Rev Dis Prim 3:17071. https://doi.org/10.1038/nrdp.2017.71
Chen J, Wang T, An C et al (2016) Brain-derived neurotrophic factor: a mediator of inflammation-associated neurogenesis in Alzheimer’s disease. Rev Neurosci 27:793–811. https://doi.org/10.1515/revneuro-2016-0017
Wang G-H, Jiang Z-L, Li Y-C et al (2011) Free-radical scavenger edaravone treatment confers neuroprotection against traumatic brain injury in rats. J Neurotrauma 28:2123–2134. https://doi.org/10.1089/neu.2011.1939
Vermersch P, Benrabah R, Schmidt N et al (2012) Masitinib treatment in patients with progressive multiple sclerosis: a randomized pilot study. BMC Neurol 12:36. https://doi.org/10.1186/1471-2377-12-36
Mora JS, Genge A, Chio A et al (2020) Masitinib as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomized clinical trial. Amyotroph Lateral Scler Front Degener 21:5–14. https://doi.org/10.1080/21678421.2019.1632346
Dubois B, López-Arrieta J, Lipschitz S et al (2023) Masitinib for mild-to-moderate Alzheimer’s disease: results from a randomized, placebo-controlled, phase 3, clinical trial. Alzheimers Res Ther 15:39. https://doi.org/10.1186/s13195-023-01169-x
Li T, Martin E, Abada Y et al (2020) Effects of chronic masitinib treatment in APPswe/PSEN1dE9 transgenic mice modeling Alzheimer’s disease. J Alzheimer’s Dis 76:1339–1345. https://doi.org/10.3233/JAD-200466
Kocic I, Kowianski P, Rusiecka I et al (2015) Neuroprotective effect of masitinib in rats with postischemic stroke. Naunyn Schmiedebergs Arch Pharmacol 388:79–86. https://doi.org/10.1007/s00210-014-1061-6
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535–b2535. https://doi.org/10.1136/bmj.b2535
Arksey H, O’Malley L (2005) Scoping studies: towards a methodological framework. Int J Soc Res Methodol 8:19–32. https://doi.org/10.1080/1364557032000119616
Tricco AC, Lillie E, Zarin W et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169:467–473. https://doi.org/10.7326/M18-0850
Hamad AA (2023) Reconsidering the need for de-duplication prior to screening in systematic reviews. AlQ J Med Appl Sci 6:367–368. https://doi.org/10.5281/zenodo.8126972
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ l4898. https://doi.org/10.1136/bmj.l4898
Sena E, van der Worp HB, Howells D, Macleod M (2007) How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci 30:433–439. https://doi.org/10.1016/j.tins.2007.06.009
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetic R, Currie M, Lisy K, Qureshi R, Mattis P, Mu P-F (2020) Chapter 7: Systematic reviews of etiology and risk. In: JBI manual for evidence synthesis. JBI. https://doi.org/10.46658/JBIMES-20-08
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from https://handbook-5-1.cochrane.org/
Piette F, Belmin J, Vincent H et al (2011) Masitinib as an adjunct therapy for mild-to-moderate Alzheimer’s disease: a randomised, placebo-controlled phase 2 trial. Alzheimers Res Ther 3:16. https://doi.org/10.1186/alzrt75
Vermersch P, Brieva-Ruiz L, Fox RJ et al (2022) Efficacy and safety of masitinib in progressive forms of multiple sclerosis. Neurol - Neuroimmunol Neuroinflammation 9:e1148. https://doi.org/10.1212/NXI.0000000000001148
Mora JS, Bradley WG, Chaverri D et al (2021) Long-term survival analysis of masitinib in amyotrophic lateral sclerosis. Ther Adv Neurol Disord 14:175628642110303. https://doi.org/10.1177/17562864211030365
Salvado M (2015) Autoimmune-like hepatitis during masitinib therapy in an amyotrophic lateral sclerosis patient. World J Gastroenterol 21:10475. https://doi.org/10.3748/wjg.v21.i36.10475
Qian J, Tu H, Zhang D et al (2021) Therapeutic effects of masitinib on abnormal mechanoreception in a mouse model of tourniquet-induced extremity ischemia-reperfusion. Eur J Pharmacol 911:174549. https://doi.org/10.1016/j.ejphar.2021.174549
Trias E, Ibarburu S, Barreto-Núñez R et al (2016) Post-paralysis tyrosine kinase inhibition with masitinib abrogates neuroinflammation and slows disease progression in inherited amyotrophic lateral sclerosis. J Neuroinflammation 13:177. https://doi.org/10.1186/s12974-016-0620-9
Trias E, Ibarburu S, Barreto-Núñez R et al (2017) Evidence for mast cells contributing to neuromuscular pathology in an inherited model of ALS. JCI Insight 2. https://doi.org/10.1172/jci.insight.95934
Trias E, King PH, Si Y et al (2018) Mast cells and neutrophils mediate peripheral motor pathway degeneration in ALS. JCI Insight 3. https://doi.org/10.1172/jci.insight.123249
Trias E, Kovacs M, King PH et al (2020) Schwann cells orchestrate peripheral nerve inflammation through the expression of CSF1, IL-34, and SCF in amyotrophic lateral sclerosis. Glia 68:1165–1181. https://doi.org/10.1002/glia.23768
Harrison JM, Rafuse VF (2020) Muscle fiber-type specific terminal Schwann cell pathology leads to sprouting deficits following partial denervation in SOD1G93A mice. Neurobiol Dis 145:105052. https://doi.org/10.1016/j.nbd.2020.105052
Ehrenreich H, Sirén A-L (2001) Neuroprotection - what does it mean? - what means do we have? Eur Arch Psychiatry Clin Neurosci 251:149–151. https://doi.org/10.1007/s004060170034
Yang L, Dong Y, Wu C et al (2021) Effects of prenatal photobiomodulation treatment on neonatal hypoxic ischemia in rat offspring. Theranostics 11:1269–1294. https://doi.org/10.7150/thno.49672
Soria JC, Massard C, Magné N et al (2009) Phase 1 dose-escalation study of oral tyrosine kinase inhibitor masitinib in advanced and/or metastatic solid cancers. Eur J Cancer 45:2333–2341. https://doi.org/10.1016/j.ejca.2009.05.010
Dubreuil P, Letard S, Ciufolini M et al (2009) Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS ONE 4:e7258. https://doi.org/10.1371/journal.pone.0007258
Hahn KA, Oglivie G, Rusk T et al (2008) Masitinib is safe and effective for the treatment of canine mast cell tumors. J Vet Intern Med 22:1301–1309. https://doi.org/10.1111/j.1939-1676.2008.0190.x
Smrkovski OA, Essick L, Rohrbach BW, Legendre AM (2015) Masitinib mesylate for metastatic and non-resectable canine cutaneous mast cell tumours. Vet Comp Oncol 13:314–321. https://doi.org/10.1111/vco.12053
Adenis A, Blay J-Y, Bui-Nguyen B et al (2014) Masitinib in advanced gastrointestinal stromal tumor (GIST) after failure of imatinib: a randomized controlled open-label trial. Ann Oncol 25:1762–1769. https://doi.org/10.1093/annonc/mdu237
Deplanque G, Demarchi M, Hebbar M et al (2015) A randomized, placebo-controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer. Ann Oncol 26:1194–1200. https://doi.org/10.1093/annonc/mdv133
Fakhoury M (2015) Role of immunity and inflammation in the pathophysiology of neurodegenerative diseases. Neurodegener Dis 15:63–69. https://doi.org/10.1159/000369933
McCauley ME, Baloh RH (2019) Inflammation in ALS/FTD pathogenesis. Acta Neuropathol 137:715–730. https://doi.org/10.1007/s00401-018-1933-9
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimer’s Dement 12:719–732. https://doi.org/10.1016/j.jalz.2016.02.010
Chu F, Shi M, Zheng C et al (2018) The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neuroimmunol 318:1–7. https://doi.org/10.1016/j.jneuroim.2018.02.015
Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14:463–477. https://doi.org/10.1038/nri3705
El Waly B, Macchi M, Cayre M, Durbec P (2014) Oligodendrogenesis in the normal and pathological central nervous system. Front Neurosci 8. https://doi.org/10.3389/fnins.2014.00145
Kwon HS, Koh S-H (2020) Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener 9:42. https://doi.org/10.1186/s40035-020-00221-2
Soltani Khaboushan A, Yazdanpanah N, Rezaei N (2022) Neuroinflammation and proinflammatory cytokines in epileptogenesis. Mol Neurobiol 59:1724–1743. https://doi.org/10.1007/s12035-022-02725-6
Woodburn SC, Bollinger JL, Wohleb ES (2021) The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J Neuroinflammation 18:258. https://doi.org/10.1186/s12974-021-02309-6
Jones MK, Nair A, Gupta M (2019) Mast cells in neurodegenerative disease. Front Cell Neurosci 13. https://doi.org/10.3389/fncel.2019.00171
Teleanu DM, Niculescu A-G, Lungu II et al (2022) An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int J Mol Sci 23:5938. https://doi.org/10.3390/ijms23115938
Ribatti D (2015) The crucial role of mast cells in blood–brain barrier alterations. Exp Cell Res 338:119–125. https://doi.org/10.1016/j.yexcr.2015.05.013
Ettcheto M, Cano A, Sanchez-López E et al (2021) Masitinib for the treatment of Alzheimer’s disease. Neurodegener Dis Manag 11:263–276. https://doi.org/10.2217/nmt-2021-0019
Ketabforoush AHME, Chegini R, Barati S et al (2023) Masitinib: the promising actor in the next season of the amyotrophic lateral sclerosis treatment series. Biomed Pharmacother 160:114378. https://doi.org/10.1016/j.biopha.2023.114378
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Conceptualization, A.A.H.; methodology, A.A.H.; screening, Y.H. and M.A.M.; data extraction, Y.H. and M.A.M.; quality assessment, B.E.A. and A.A.H.; drafting the manuscript, A.A.H., B.E.A., and M.M.; figures creation, A.A.H.; critical revision, A.A.H.; administration, A.A.H. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethics approval
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamad, A.A., Amer, B.E., Hawas, Y. et al. Masitinib as a neuroprotective agent: a scoping review of preclinical and clinical evidence. Neurol Sci 45, 1861–1873 (2024). https://doi.org/10.1007/s10072-023-07259-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-07259-w