Abstract
Behavioral coordination is involved in many forms of primate interactions. Co-representation is the simultaneous mental representation of one’s own and the partner’s task and actions. It often underlies behavioral coordination and cooperation success. In humans, the dyadic social context can modulate co-representation. Here, we first investigated whether individual differences in co-representation in the joint Simon task in capuchin monkeys and Tonkean macaques can be explained by social factors, namely dyadic grooming and sociality index, rank difference and eigenvector centrality. These factors did not predict variation in co-representation. However, in this specific task, co-representation reduces rather than facilitates joint performance. Automatic co-representation therefore needs to be inhibited or suppressed to maximize cooperation success. We therefore also investigated whether general inhibitory control (detour-reaching) would predict co-representation in the joint Simon task in Tonkean macaques, brown capuchin and marmoset monkeys. Inhibitory control did neither explain individual differences nor species differences, since marmosets were most successful in their joint performance despite scoring lowest on inhibitory control. These results suggest that the animals’ ability to resolve conflicts between self and other representation to increase cooperation success in this task is gradually learned due to frequent exposure during shared infant care, rather than determined by strong general inhibitory control. Further, we conclude that the joint Simon task, while useful to detect co-representation non-invasively, is less suitable for identifying the factors explaining individual differences and thus a more fruitful approach to identify these factors is to design tasks in which co-representation favors, rather than hinders cooperation success.
Similar content being viewed by others
Introduction
Various natural forms of human and nonhuman primate cooperation like coalition formation, food sharing, group hunting, territorial defense, biparental care, communication or social play involve behavioral coordination with other individuals (Burkart et al. 2022; Clark 2006; Hrdy 2009; de Waal and Suchak 2010; Tomasello 2019; Heesen et al. 2021). Fine-tuned motor coordination can be enabled through the mental representation of not only one’s own but also the partner’s task and actions (Sebanz et al. 2003, 2005; Vesper et al. 2010, 2017). Co-representation involves basic perception–action matching (ideomotor theory or common coding, Prinz 1997; Hommel et al. 2001) and action simulation (simulation theory, Gallese and Goldman 1998) when individuals internally simulate actions performed by the co-actor and integrate this simulation with representations of own action goals and planned subsequent actions (Sebanz et al. 2007; Bekkering et al. 2009; Knoblich et al. 2011). As a part of self–other (SO) integration, this co-representation presumably facilitates joint performance because it allows for immediate predictions of the partners’ behaviors and enables individuals to prepare their actions in anticipation of their partner’s actions, and therefore refines motor coordination (Sebanz et al. 2006a; Sommerville and Decety 2006; Vesper et al. 2010; Butterfill 2012; Ruissen and de Bruijn 2016). Co-representation is involved in basic dyadic motor coordination requiring complementary actions, such as the joint Simon task (in humans: Sebanz et al. 2003; Ruys and Aarts 2010; in primates [platyrrhine and catarrhine monkeys]: Miss et al. 2022; Miss and Burkart 2018) or joint music performance (Novembre et al. 2016; see also vocal turn-taking in cooperative communication in callitrichids: Takahashi et al. 2013). Co-representation is particularly useful when individuals perform identical actions simultaneously and successful coordination increases with motor alignment through self-other (SO) integration, such as in joint grasping/lifting/pulling tasks or synchronization tasks (Newman-Norlund et al. 2008; Vesper et al. 2013, 2014; Meyer et al. 2016; Schmitz et al. 2017; in primates [chimpanzees]: Melis et al. 2006; Constable et al. 2021).
In human adults, some evidence suggests that social factors can modulate co-representation. For instance, co-representation emerged only with a friendly acting, supportive partner but not with an intimidating, competitive partner (Hommel et al. 2009), and between in-group (i.e., partners belonging to the same group) but not out-group members in case of a salient group categorization (McClung et al. 2013; see Iani et al. 2011 for no modulation with a minimal group categorization). Likewise, a competitive rather than cooperative set-up between the co-actors either in the joint Simon task (Iani et al. 2011) or in an unrelated dyadic task preceding the joint Simon task (Iani et al. 2014; Ruissen and de Bruijn 2016) hindered the emergence of co-representation. Moreover, co-representation increased with perceived inter-personal closeness between partners (assessed with the Inclusion of the Other in the Self scale, Shafaei et al. 2020). Intriguingly, co-representation even emerged with an invisible co-actor, if the partner believed that the complementary motor responses came from a social, intentionally acting partner instead of being automatically generated by an algorithm (Tsai et al. 2008; Sahaï et al. 2019). A first goal of our study was therefore to investigate whether and how social variables predict the co-representation previously reported in dyads of common marmosets (Callithrix jacchus), capuchin monkeys (Sapajus apella) and Tonkean macaques (Macaca tonkeana) tested with a joint Simon task (Miss et al. 2022; Miss and Burkart 2018).
The smooth motor coordination between individuals does not merely depend on strong SO integration, but also requires an optimal balancing between SO integration vs. SO distinction (e.g., Steinbeis 2016). Studies on neural inter-brain underpinnings of social interaction during real-time joint action tasks showed that co-actors’ brains are linked through coupled neural oscillations, for instance, during joint rhythmic behavior, joint speech, or joint movement behavior (Keller et al. 2014; Novembre et al. 2016; Djalovski et al. 2021). In particular, these inter-brain neural processes appear to play a crucial role in the regulation between SO integration (and social alignment) and SO distinction (Novembre et al. 2016; Gvirts and Perlmutter 2020; de Hamilton 2021). This distinction is most necessary in contexts when co-representation hinders rather than facilitates joint performance and thus cooperation success, as for instance, in joint interference tasks like the joint Simon task (Sebanz et al. 2003) and the joint flanker task (Atmaca et al. 2011), or imitation-inhibition tasks (Spengler et al. 2010), or perspective-taking tasks (Samson et al. 2010). Since co-representation and SO integration most likely emerge as an automatic process in these contexts (Decety and Sommerville 2003; Brass et al. 2009; see also Southgate 2020; Sebanz and Knoblich 2021), SO distinction requires active inhibition and suppression of co-representation. Our second goal of this study was therefore to investigate how primate co-representation in the joint Simon task is linked to individual differences in independently assessed inhibitory control.
The joint Simon task (Sebanz et al. 2003) is a basic dyadic motor coordination task where co-representation hinders joint performance and cooperation success. It has the advantage of making co-representation visible and thus allowing a non-invasive, behavior-based quantification of co-representation (e.g. in nonhuman primates, Miss et al. 2022; Miss and Burkart 2018). However, it is a joint interference task and therefore integrating the co-actor’s action and task requirements makes the distinction between one’s own and the partner’s task affordances more difficult. Therefore, joint performance success in this task crucially requires SO distinction.
The primate task design (Miss and Burkart 2018) adopted the auditory version of the joint Simon task from human studies (Sebanz et al. 2003; Ruys and Aarts 2010). In this experimental paradigm, two individuals share the task to correctly react to an external sound stimulus by choosing one of two response options, each individual being responsible for one of them. In particular, two different sounds require either answering on the left-hand or on the right-hand side of a response device (e.g. sound A requires answering on the left-hand side and sound B requires answering on the right-hand side) and in case of a correct choice, both partners receive a reward, independent of which actor provided it. This task set-up is similar to the cooperative designs used with human adults, in which dyads are explicitly instructed to cooperate, sometimes emphasized by the promise that the best performing dyads in a given group of participants will receive a reward (e.g., Tsai et al. 2008; Ruys and Aarts 2010; Iani et al. 2014).
The difficulty of the joint Simon task lies in the directional properties of the sounds, namely, the sounds are broadcast from either the left-hand or the right-hand direction, which creates either compatible trials (i.e., the side of the broadcast and the answer match) or incompatible trials (i.e., the side of the broadcast is opposite to the answer side). Typically, answering to incompatible stimuli is more difficult than answering to compatible ones when individuals share the task with a joint action partner (i.e., when one individual is responsible for answering on the left-hand side and the other one for answering on the right-hand side): the joint Simon effect (Sebanz et al. 2003; Ruys and Aarts 2010). Intriguingly, this Simon effect disappears when they do the exact same half part of the task on their own during an individual control condition (e.g., only answering to sound A when situated on the left-hand side). Thus, when sharing the task with a partner, typically error rates in response choices (mainly in primates) and in first heading directions (i.e., the first subtle movement or body orientation toward a response side, only measured in primates: Miss et al. 2022), and delays in reaction times (mainly in humans) are increased in incompatible trials (i.e., the joint Simon effect indicative of co-representation: Sebanz et al. 2003). To capture subtle differences in reaction times, animals would have to be trained to respond as quickly as possible. Instead, we found that errors in response choices as well as first heading directions were reliable variables to quantify co-representation in the joint Simon task in primates (Miss et al. 2022; Miss and Burkart 2018).
Since in the joint Simon task co-representation reduces rather than facilitates cooperation success (i.e., the total amount of rewards received by both), the amount of correct response choices (i.e., the cooperation success) in incompatible trials indicates how flexible co-representation is deployed to increase SO distinction and improve joint performance. Such flexibility requires the inhibition of co-representation, and electrophysiological and fMRI evidence indeed shows the recruitment of control mechanisms in the joint Simon task to inhibit motor responses when it is the partner’s turn, and to increase action monitoring and joint attentional processes (Sebanz et al. 2006b, 2007; Tsai et al. 2006; Ruissen and de Bruijn 2015). The latter is in line with the behavioral studies in primates showing a higher frequency of visual monitoring behavior directed at the partner when both individuals are engaged together in the task than when the partner is present but cannot engage in the task (i.e., blocked access to the response device; Miss et al. 2022; Miss and Burkart 2018).
Recent studies have addressed the evolutionary origin of co-representation and reported co-representation assessed with the joint Simon task in three primate species, the highly cooperative common marmosets, the intermediate brown capuchins, and the Tonkean macaques who less often engage in cooperative actions with each other (Miss et al. 2022). Common marmosets, like humans, qualify as cooperative breeders: in addition to mothers, other group members regularly contribute to rearing offspring, which increases infant growth and survival (Burkart et al. 2009; Hrdy 2009; Erb and Porter 2017). Capuchin monkeys and Tonkean macaques, in contrast, are independent breeders and the prevalence of cooperation among group members during everyday interactions is thus comparatively lower than in marmoset monkeys (Petit et al. 1992; Perry and Rose 1994; Thierry et al. 1994; Mendres and de Waal 2000; Bergstrom and Fedigan 2010; Burkart et al. 2014). Co-representation became weaker as general cooperativeness in a species increased, from Tonkean macaques, to capuchins, to marmosets. Thus, co-representation emerges in experimental contexts even in species such as the Tonkean macaque, in which dyads who are tolerant enough to engage in such a task together repeatedly are most likely less common than, for instance, in marmosets (Petit et al. 1992; Burkart et al. 2014; Martin et al. 2021). Moreover, the documented interspecific variation suggests that the more a species engages in cooperation (facilitated in particular by shared infant care among group members), the better it is to selectively suppress spontaneous co-representation if necessary (as in the joint Simon task), and therefore achieves higher cooperation success. It thus appears that primates (at least haplorrhines) generally co-represent their partners’ tasks and actions when engaged in a joint action task, but the flexibility to regulate co-representation and suppress it if this optimizes cooperation success appears higher in species who most routinely engage in joint activities during everyday life, rather than in bigger-brained species (Miss et al. 2022).
The suppression of co-representation may entirely depend on strong general inhibitory control abilities. In line with this, a study with 4–5-year-old children found that besides Theory of Mind (ToM), stronger motor inhibitory control skills (independently assessed with a modified day-night Stroop task, Gerstadt et al. 1994, and the pictures task, Burns et al. 2012) were associated with weaker co-representation in a joint action task requiring complementary actions (Milward et al. 2017). However, an exclusive reliance on general inhibitory control is not in line with the finding that among the three primate test species, brain size increases from marmosets to capuchins to Tonkean macaques (Deaner et al. 2007) and brain size and general inhibitory control tend to be correlated in primates (MacLean et al. 2014). Yet, the inhibition of co-representation was strongest in the marmosets, the species with the smallest brain.
An alternative to general inhibitory control is that the suppression of co-representation is achieved through repeatedly and frequently experiencing SO integration—distinction conflicts during joint action (cooperative flexibility hypothesis, Miss et al. 2022). Species in which group members are highly interdependent (de Oliveira Terceiro et al. 2021) and regularly engage in cooperative activities, particularly required for joint infant care (e.g., food sharing, coordination of infant transfers, group defense and vigilance or communicative exchanges, Burkart et al. 2022; Snowdon 2001; Takahashi et al. 2013; Guerreiro Martins et al. 2019; Hrdy and Burkart 2020) are likely to have recurrent opportunities to engage in joint actions and learn the necessary skills from an early age on. This may include practicing and learning to optimally balance SO integration and SO distinction during joint action and thus to selectively suppress automatic co-representation, for instance when coordinating complementary actions (e.g., handing over an infant from one carrier to another) or mutually exclusive activities among group members (e.g., feeding vs. vigilance). A training study with human subjects supports this alternative that inhibition of co-representation may be achieved independently of general motor inhibitory skills. Using a perspective-taking task (i.e., the Director’s task), Santiesteban et al. (2012) found that the prior training to inhibit imitation, but not of motor inhibitory control in general (assessed with a Stroop-like paradigm), increased the participants’ subsequent task success.
Our goal was to investigate if social factors or general inhibitory control ability could explain individual differences in co-representation in the Tonkean macaques, the brown capuchin and the common marmoset monkeys from Miss et al. (2022) and Miss and Burkart (2018). To examine the role of social factors, we collected from the Tonkean macaques [TM] and the brown capuchins [BC] observational data on social behaviors to quantify dyadic bond strengths (i.e., a dyadic grooming index (DGI) and a dyadic composite sociality index (DSI) [TM]) (Silk 2007), social rank differences (Elo-ratings) [TM, BC] (Neumann et al. 2011) and eigenvector centrality values based on the social affiliative network [TM] (Brent 2015). Individuals with high centrality values tend to have a large number of partners and more frequent affiliative interactions (Silk 2007; Cheney et al. 2016). In animals, particularly those which frequently cooperate in the wild, such as ravens, Corvus corax (Massen et al. 2015; Asakawa-Haas et al. 2016) or wolves, Canis lupus (Dale et al. 2020), dyads with stronger social bonds and closer in rank (but see Molesti and Majolo 2016 in barbary macaques, Macaca sylvanus) tend to be better cooperators in joint pulling tasks requiring simultaneous actions. Based on the findings in animals and humans, strong social bonds can be predicted to lead to stronger SO integration and co-representation. However, based on the conflicting role of co-representation and the necessity of SO distinction in the joint Simon task, we may likewise predict higher cooperation success (and thus weaker co-representation) in dyads with stronger social bonds. This is particularly likely if the suppression of co-representation does not require general inhibitory control ability but can be achieved otherwise, for instance through repeated exposure to SO conflicts in joint action contexts and training. This latter pathway would be consistent with the cooperative flexibility hypothesis, which proposes co-representation as a universal, automatic process in primates, and that the flexibility to suppress it is not primarily dependent on advanced general cognition but is enhanced when individuals are frequently exposed to SO integration—distinction conflicts.
To examine the role of general inhibitory control ability in suppressing co-representation in the joint Simon task, we tested in the Tonkean macaques, the brown capuchins and the common marmosets whether inhibitory control skills independently assessed with a detour-reaching task (MacLean et al. 2014; Schubiger et al. 2019; Gokcekus 2020) would predict individual differences in co-representation. Detour-reaching paradigms are frequently used to test the ability to inhibit or withhold pre-potent motor responses of directly reaching for an immediate apparent reward and instead make a detour (Amici et al. 2008; Vlamings et al. 2010; Manrique et al. 2013; Kabadayi et al. 2018). Since Tonkean macaques showed rather strong co-representation compared to much smaller brained common marmosets, and based on findings in humans (Santiesteban et al. 2012), we did not expect that general motor inhibition ability would explain variation in the strength of co-representation in these monkeys. Indeed, the species differences (Miss et al. 2022) suggest that stronger general inhibitory control might not primarily predict the ability to selectively suppress co-representation to improve cooperation success in the joint Simon task, whereas the habitual engagement in joint activities (cooperative flexibility hypothesis, Miss et al. 2022) does.
Methods
Strength of co-representation
Data on individual differences in co-representation during the joint task were taken from an auditory version of the joint Simon task and available from nine marmosets, six capuchins and seven macaques (Miss et al. 2022; Miss and Burkart 2018). The same response device (adjusted to body size), training and testing procedure and test criteria were applied in the three species. The response device consisted of two sliding drawers, which could be pulled within reach with fixed handles, and contained two fixed cups each (one outer cup for the focal individual answering to the stimulus and one cup in the middle for the partner individual). The monkeys could swing the cups open to retrieve a food reward simultaneously in case of a correct choice (Fig. 1). The two drawers were connected with a cord going around a pole in the back. This mechanism moved the second drawer backwards and out of reach for the partner monkey as soon as one drawer was pulled. In the training phase the subjects were alone and the two sounds were broadcast from the middle (thus not creating any stimulus incompatibility), and the monkeys learned the association between the sound stimulus and the corresponding response side (the left-hand or the right-hand drawer). The individual learning criterion was to reach at least six sessions consisting of 12 trials with at least 75% correct choices. After subsequently passing the criterion of 75% correct choices in a joint task set-up, the individuals were then tested in the joint task condition in five sessions (respectively four in case of the two marmoset breeding pairs) consisting of 12 trials each. Differences across individuals in previous participation in cognitive tasks and in motivation were thus controlled to some degree by the requirement of passing several criteria (see also below), in particular to have learned the association between the sounds and sides. This precondition ensured that all individuals were motivated to participate in the tests and sufficiently attentive to the stimuli to learn the association and the task demands.
Experimental set-up describing the joint Simon task condition. The apparatus consisted of two sliding drawers with fixed handles to pull the drawers within reach and retrieve a food reward out of the cups in case of a correct choice. The focal individual could retrieve its food reward out of the outer cup (O) and the partner monkey out of the cup in the middle (M). In every trial, one of the two auditory stimuli “A” or “B” were broadcast from one of the two lateral speakers. Sound “A” asked for pulling the drawer on the left-hand side, whereas sound “B” asked for pulling the drawer on the right-hand side. The set-up was the same in all three test species
The training and testing procedure were identical. A separation grid was placed between the individual and its partner, allowing constant visual contact of the monkeys with each other. A screen permitted baiting of the cups out of sight of the monkeys and ensured that the drawers remained out of reach before the start of a trial. While attracting the subjects’ attention to the middle in front of the testing device, a trial started with the broadcast of the sound. Simultaneously, the screen was lifted and the two drawers were pushed within reach of the subjects. A trial ended when one of the two subjects pulled one of the two drawers, thus requiring an exclusive choice in each trial. In case of a correct choice, the subjects could retrieve the reward simultaneously from the outer and middle cup, respectively (i.e., joint reward) and consume it, while the other two cups were opened to uncover the non-baited side. In case of an incorrect choice, the non-selected cups were opened to uncover the baited side and the screen was lowered.
A test session started with two to six motivation pre-trials in which the sounds were broadcast from a central position (identical to the training phase) to ensure that the subjects remembered the corresponding sound-side association and to check their motivation of receiving food items as rewards. The test session was only started on a particular day if they chose each baited side twice correctly during these pre-trials. If this criterion was not met, the subjects were tested the following day. In every test trial, the sounds were played back from either the left- or the right-hand side, thus either eliciting stimulus incompatibility or not. In compatible trials, sound “A” was played back from the left-hand side and sound “B” from the right-hand side, whereas in incompatible trials, sound “A” was played back from the right-hand side and sound “B” from the left-hand side. The order of the two sounds and of the sides from which they were broadcast as well as the sides of the subjects were pseudo-randomized and counterbalanced. Since in the joint task, the individuals shared the task with a partner monkey, we expected a joint Simon effect (i.e., co-representation) in the focal individual, expressed in increased error rates in incompatible trials and consequently fewer joint rewards.
In the marmosets, the dyads were formed within their family groups (or consisted of the breeding pair, respectively) and preselected according to observations of affiliative behaviors during the training phase, such as entering together and remaining in proximity in the testing cage, participating next to each other in trials and occasionally food sharing. The composition of the dyads was the same over all sessions. In the brown capuchins and the Tonkean macaques, the dyads were not predefined but formed in every session according to which animals decided to enter the experimental facility together at the same time. According to this free partner choice method, the composition of the dyads could vary across sessions but the dyads contained only partners that tolerated each other’s close proximity in the context of receiving food simultaneously. In both species, this resulted in pairings with the same partner individual in at least two sessions (four individuals in the capuchin group even had the same partner in four or all sessions). Table 1 shows a description of all tested individuals.
Observations of social behaviors
Subjects
We collected behavioral data on 22 individuals (adults > 6 years, sub-adults > 4 years and < 6 years) in a group of semi-free ranging Tonkean macaques (Macaca tonkeana). The group consisted of 28 individuals during the period of data collection: 18 adults (9 females), four sub-adults (all males), five juveniles (< 4 years, too young to be reliably identified during the period of data collection) and one newborn. We also observed 14 individuals in a group of semi-free ranging brown capuchins (Sapajus apella) which consisted of 19 individuals during the study period: six adults (five females), six sub-adults (two females), five juveniles (three females) and two newborns. All animals were housed at the Primate Centre of the University of Strasbourg, France and were captive born. The Tonkean macaques and the capuchin monkeys both had permanent access to a wooded park of 3788 m2 and 2332 m2 respectively, connected to a heated indoor-outdoor shelter. They were provisioned with commercial primate pellets twice a day and with fresh fruit and vegetables once a week. Water was available ad libitum. The Tonkean macaques moreover had 24 h access to Machines for Automated Learning and Testing (MALT) with the option to perform several cognitive tasks (Fizet et al. 2017). The observations were conducted non-invasively and the study was performed according to the French legal requirements for the use of animals in research and complied with the EU Directive 2010/63/EU on the welfare of animals used for scientific purposes.
Data collection
To test for effects of the social relationships and dominance hierarchy on the joint Simon effect, we recorded affiliative and agonistic interactions in the Tonkean macaque group during focal samplings and ad libitum observations (Altmann 1974). Prior to the start of the observational data collection, the animals were habituated to the researcher’s presence inside their park for two weeks. Subsequently, behavioral data on the Tonkean macaque group were collected by two researchers (BS & FM) right before the start of the Simon task study (March 14 until July 5, 2018) and immediately after (November 13 until December 13, 2018), providing observational data from 5 months. This resulted in approximately 6.6 h of observation time per individual (mean ± SEM = 6.6 h ± 0.1 h), and 769 recorded conflictual events. A part of these data have already been used in another study (Ballesta et al. 2021).
All adult and sub-adult members of the group were studied with the focal sampling method during 10 min sessions. One adult male (‘Wotan’) was removed from the group on June 1, 2018 and transferred to another park. Therefore, the total amount of observational time was slightly lower (5 h) for this individual compared to the others. Observations were only recorded when the individual was in complete view (in the park or the outdoor shelter) and were balanced evenly throughout the day (8 h 30–18 h). An individual was observed only once a day and the order of the focal follows was assigned randomly every day. If the focal animal could not be located, the next assigned individual was observed instead. No more than four individuals from the assigned order could be skipped to avoid recording when most group members were in the indoor shelter or out of view. Focal samplings were only used for analyses if the total time during which the focal animal was in view was at least 5 min. Toward the end of the observational periods, specific individuals were prioritized to correct for unbalanced distribution of observational sessions across time.
Behaviors were defined based on the social repertoire of Tonkean macaques (Thierry et al. 1990, 2000). Dyadic affiliative interactions included social grooming (s) and social contact (s) defined as two individuals sitting next to each other with body contact. They were only recorded during the focal follows, while aggressive and submissive interactions were additionally recorded ad libitum. Agonistic interactions were described as physical conflicts (e.g., wrestles, bites, slaps), chases, threats (e.g., open-mouth threats, stamps, stares), and displacements (the arrival of an individual is followed by the immediate departure of the approached individual, e.g., from a resource such as food or a consorted female or MALT). Submissive behaviors in the context of agonistic interactions included flights, crouching, moving away, and screams, potentially combined with facial expressions (e.g., silent-bared teeth). The actor, receiver and possible retaliation were recorded for every agonistic interaction (see supplementary material on hierarchy analysis).
Behavioral observations were recorded either on paper or on an IPod Touch with the Animal Pro Behavior software (Newton-Fischer, University of Kent 2012). Based on an entire week of behavioral observations (total of 89 focal follows), inter-observer reliability between BS and FM was calculated on the durations (s) of observed social grooming and social contact behavior (ICC = 0.99) and on the recorded agonistic events as well as the identities of the observed individuals (Cohen’s κ = 0.89).
Data analysis
Hierarchy analysis
All the statistical analyses were conducted in R (version 3.5.3). With the Tonkean macaque data, we calculated Elo-ratings (Neumann et al. 2011) and David’s scores (de Vries et al. 2006) with the package ‘EloRating’ and rank stabilities with the package ‘Perc’ (Fujii et al. 2019) from a sequence of agonistic interactions recorded during the observational periods (Vilette et al. 2020) (see supplementary material on hierarchy analysis, Table S1 and S2). We then used the ranking output based on the Elo-ratings to define the dominance hierarchy and entered the scores of the individual’s absolute difference in rank compared to its joint action partner as fixed factors in the model calculation.
We could not base the dominance ranking for the capuchin monkeys on quantified data due to a lack of available behavioral data within the composition of the group during the Simon task test period from Miss et al. (2022). Instead, two observers estimated the hierarchy through ad libitum observations of agonistic interactions describing dyadic (physical) aggression and submissive behavior in resource related and social contexts, such as spatial displacements, supplants, avoidances, flights and screams (Leca et al. 2002; Bergstrom and Fedigan 2010).
Dyadic grooming index, dyadic composite sociality index and social network analysis in the Tonkean macaques
We turned all behavioral data into rates and included only data of the adult and sub-adult group members in the analyses (n = 22). For the observed dyads in the joint task, we calculated dyadic grooming indices (DGI) and dyadic composite sociality indices (DSI) based on the durations of the behaviors grooming and sitting in contact (Silk et al. 2013). DGI(xy) were calculated as the total grooming time of a dyad xy divided by the mean grooming time across all dyads. DSI(xy) were calculated as follows:
while \({f}_{ixy}\) is the total time of grooming or sitting in contact for dyad xy and \({\overline{f} }_{i}\) is the mean grooming time or the mean sitting in contact time across all dyads. Therefore, high values of the DGI and the DSI represent dyads that had more frequent and/ or longer lasting affiliative interactions than the average dyad in the group and low values represent dyads which had less frequent and/ or shorter affiliative interactions than the average dyad.
To analyze a potential link between the subjects’ positions in an affiliative network of the group and their joint Simon effects, we further used the durations of the grooming and social contact behaviors to calculate eigenvector centrality measures with social network analysis (Brent 2015) using the package “igraph” (supplementary material, Fig. S1).
Model calculation
We calculated binomial generalized linear mixed effect models (glmm) using the package “lme4” with the Tonkean macaque and brown capuchin data to analyze a potential influence of social factors on the subjects’ strength of co-representation (i.e., joint Simon effect). Accordingly, to test for a potential influence of social factors on the Tonkean macaques’ joint Simon effects, we built a model on the joint task data with choice (either correct or incorrect answer) as a binary response variable and compatibility, absolute rank difference, DGI, DSI and eigenvector centrality as fixed factors. To test for a potential influence of social rank differences on the capuchins’ joint Simon effect, we built a model on the joint task data with choice as a binary response variable and compatibility and absolute rank difference as fixed factors. Both models included individual, session and partner as random factors, and were compared to a control model, containing only the control factor (compatibility) and the random factors. For all statistical analyses, model parameters were approximated using maximum likelihood estimation and model performance was assessed by likelihood ratio tests. All figures were generated using the package “ggplot2”.
Inhibitory control
Subjects
In the detour-reaching task, we tested seven Tonkean macaques and five brown capuchins from the same study groups, and added the data of five common marmosets (Callithrix jacchus) tested with the identical detour-reaching task (Gokcekus 2020). All individuals also participated in the joint Simon task studies from Miss et al. (2022) and Miss and Burkart (2018). The marmosets were captive-born and housed in family groups at the Primate Station of the University of Zurich, Switzerland in heated indoor enclosures with access to outdoor enclosures during appropriate outdoor temperatures (> 10 °C). They were provisioned with mash and fresh fruit and vegetables every day. Water was available ad libitum. The research was approved by the Kantonales Veterinäramt, license number 223/16. For all study groups, subject participation was voluntary, the normal feeding routine was maintained during testing and animals were never food or water deprived.
Procedure
We measured inhibitory control ability with a detour-reaching task adapted for primates (MacLean et al. 2014; Schubiger et al. 2019). The task required the individual to reach around a transparent barrier (Plexiglas panel: 25 cm × 25 cm for the Tonkean macaques, 18 cm × 18 cm for the capuchin monkeys and 8 cm × 8 cm for the marmoset monkeys) to retrieve a food item placed behind it (Fig. 2). The panel was vertically attached to the top of a wooden board. We conducted five sessions consisting of 12 trials (60 trials in total). In each session, the reward appeared four times in each of three possible locations (central = fully behind the panel; left = half exposed; and right = half exposed) in a counterbalanced and pseudo-randomized order with the rule that the reward never appeared in the same location in more than two consecutive trials. In the more difficult central trials, the reward was placed in the middle of the Plexiglas barrier so that it was fully occluded by it. The two lateral conditions served as a distraction and an attenuation of the level of difficulty since the subject could directly reach for the food item.
In a familiarization phase, every individual was given the opportunity to explore the Plexiglas panel through the grid for 10 min without a food reward present. In the test trials, we first placed a cardboard screen between the grid and the panel to occlude the positioning of the food item in one of the three locations (i.e., central, left or right). As soon as the individual was attentive, we called her name and removed the cardboard screen. In case the first attempt was not successful, we kept the panel in place for max. 2 min to allow further attempts to reach around the panel and retrieve the food item. The individuals completed a session on the same day in as many trial-blocks as needed. All trials were video-recorded.
Data analysis
We scored all trials from the videos as either a successful first attempt (i.e., directly reaching around the panel) or not (i.e., reaching into the panel first) and calculated the measure of successful detour-reaching per individual as the percentage of correct trials at first attempt out of the 20 central trials. We assessed interrater reliability (Cohen’s κ = 0.97) for successful first attempts in the Tonkean macaques and the brown capuchins with 20% randomly selected video sessions. For the model calculations, we built glmms using the package “lme4” with the Tonkean macaque, the brown capuchin, and the marmoset data to analyze a potential influence of inhibitory control ability on the individuals’ strength of co-representation (i.e., joint Simon effect). Accordingly, we built models on the joint task data with choice as a binary response variable and compatibility and inhibitory control as fixed factors. The models included individual (nested in species in the analysis across the three species), session and partner as random factors, and were compared to a control model, containing only the control factor (compatibility) and the random factors.
For species comparisons in inhibitory control ability, we calculated a binomial glmm with the individual detour-reaching scores (either successful or failed first attempt) as binary response variable and all variables of interest (species, session, age, sex) as fixed factors. This model was compared to the null model consisting of the intercept and random effects only. Individual nested in species, and session were included as random factors. For the fixed factor session, we set a priori polynomial contrast to test for trends across time. For pairwise comparisons between species, we performed Tukey adjusted post hoc tests using the package “emmeans”. Variance Inflation Factors (VIFs) were calculated to examine if predictors did not violate any multicollinearity assumptions using the package “car” (all VIF scores < 2). The proportion of the total variance accounted for by the model was assessed by the conditional R2GLMM value using the package “MuMIn”. For all statistical analyses, model parameters were approximated using maximum likelihood estimation and model performance was assessed by likelihood ratio tests. All figures were generated using the package “ggplot2”.
Results
An influence of social factors on the joint Simon effect?
We tested if social factors estimated with the social rank difference (Elo-ratings), the integration in the affiliative social network (eigenvector centrality values), and the bond strength with a given partner (the dyadic grooming index (DGI) and the dyadic composite sociality index (DSI)) could explain variation in co-representation in the Tonkean macaques and the brown capuchin monkeys. We built models on the joint Simon task data with choice as a binary response variable and found that the models did not improve compared to the control models containing only the fixed factor compatibility and the random factors [Tonkean macaques: \(\chi_{4}^{2}\) = 2.64, p > 0.05, ∆AIC = 5.36, Ntotal = 209, Nindividuals = 7; brown capuchins: \(\chi_{1}^{2}\) = 0.13, p > 0.05, ∆AIC = 1.87, Ntotal = 184, Nindividuals = 6]. In both models, only the fixed factor compatibility remained as a significant predictor, and none of the other fixed factors (absolute rank difference, eigenvector centrality, DGI, DSI) showed a significant effect (Table 2).
Therefore, individuals with partners of similar rank, or highly connected individuals with a large number of affiliative well-connected partners, or individuals with more strongly bonded partners generally did not show weaker or stronger co-representation (Table 2, Fig. 3 and supplementary material Fig. S2 & S3).
Relationship between the integration in the social network and co-representation. The strength of co-representation (i.e., joint Simon effect; % incorrect choices in incompatible minus compatible trials per session) in the Tonkean macaques is shown according to an individual’s eigenvector centrality value from low (light shading) to high (dark shading). The boxes and whiskers represent medians and lower and upper quartile scores. Error bars represent standard errors of the mean. More peripheral individuals in the affiliative social network and more central individuals did not differ in the strength of their co-representation (p > 0.05)
Association between inhibition (detour-reaching) and the joint Simon effect?
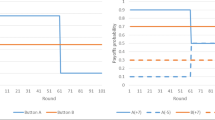
Better inhibitory control ability assessed with a detour-reaching task was not associated with weaker (or stronger) co-representation (i.e., smaller or larger joint Simon effect), neither when analyzed across all individuals [\(\chi_{1}^{2}\) = 0.13, p > 0.05, ∆AIC = 1.87, Ntotal = 686, Nindividuals = 17] nor when analyzed within each species (Table 3, Fig. 4).
Relationship between inhibitory control ability and co-representation. Every individual’s joint Simon effect measure (i.e., the observed difference of incorrect choices between incompatible and compatible trials) is shown in relation to its inhibitory control measure (i.e., observed correct inhibition trials at first attempt in the detour-reaching task) in the Tonkean macaques (n = 7), the brown capuchins (n = 5), and the common marmosets (n = 5). The regression lines are the correlations between joint Simon effect measures and inhibitory control measures per species (solid) and overall (dashed) and do not show model predictions. The shaded areas display 95% confidence intervals
When testing for species differences in inhibitory control ability, the full model explained the data better than the null model [\(\chi_{8}^{2}\) = 21.48, p = 0.006, ∆AIC = 5.48, Ntotal = 340, Nindividuals = 17] and revealed a significant effect of species and session (supplementary material, Table S3 and Fig. S4). The monkeys improved their inhibitory control score over time (liner trend of session: β (SE) = 1.61 (0.39), 95% CI = [0.84, 2.38], z = 4.10, p < 0.001). The capuchin monkeys generally had the highest inhibitory control scores (mean ± SEM = 82.00% ± 2.00%), followed by the Tonkean macaques (mean ± SEM = 23.57% ± 5.85%) and the common marmosets (mean ± SEM = 17.00% ± 17.00%). Pairwise Tukey adjusted post hoc tests revealed that the inhibitory control scores were significantly higher in the capuchins than in the marmosets (β (SE) = 5.11 (1.33), 95% CI = [1.99, 8.24], z = 3.83, p < 0.001) and higher than in the Tonkean macaques (β (SE) = 3.27 (1.01), 95% CI = [0.91, 5.63], z = 3.24, p = 0.003). No difference was found between the marmosets and the Tonkean macaques (β (SE) = − 1.85 (1.24), 95% CI = [− 4.75, 1.06], z =− 1.49, p = 0.30) (Fig. 4).
Discussion
Individual variation in co-representation in the joint Simon task in Tonkean macaques, capuchin monkeys and common marmosets were not predicted by various social factors representing dyadic bond strength, social rank differences and connectedness in the affiliative social network, or general inhibitory control ability as measured with a detour-reaching task. Several explanations may account for these results, as we will discuss in detail below. First, the variation in social bond strength may have been too small in the primate data containing only tolerant dyads. Second, the specific affordances of the joint Simon task may have masked an effect of social bond strength. If a stronger bond between partners increased spontaneous co-representation, but also increased the effort put into maximizing cooperation success by suppressing co-representation, the net effect may not be visible because these opposing effects cancel each other out. This is particularly likely if general inhibitory control is not the main factor determining the strength of co-representation, which was indeed not the case in the primates tested here. The overall pattern thus appears most consistent with the cooperative flexibility hypothesis, namely that co-representation is an automatic default mechanism readily emerging when primates tolerantly engage in joint activities, but that the flexibility to balance SO integration vs. distinction requires practice and is therefore highest in those species routinely cooperating which is facilitated in particular by shared infant care.
A first explanation for the absence of a relationship between co-representation and social factors in the primates tested here may be that the variation in social bond strength was not large enough to detect any effect. To be able to conduct the joint Simon task at all in the capuchin monkeys and the Tonkean macaques, the dyads were formed in every session according to the animals coming voluntarily inside the experimental facility together at the same time. We therefore could only test highly tolerant dyads and lack strong social contrasts, such as co-representation in non-tolerant dyads, and dyads that are composed of different groups (in-group—out-group effects). In the marmosets, all dyads of a group are generally tolerant enough to be tested together, thus leading to ceiling effects regarding social tolerance. This first explanation is not unlikely because in humans, the influence of social factors on co-representation is often only observable in strong social contrasts. In particular, co-representation was observed in a cooperative but not a competitive task framing (Iani et al. 2011, 2014; Ruissen and de Bruijn 2016) and between in-group but not out-group members (Müller et al. 2011; McClung et al. 2013) but only if the group categorization was made highly salient (Iani et al. 2011). Further, it emerged only with a friendly acting, supportive partner but not with an intimidating, antagonistic partner (Hommel et al. 2009). It thus seems that task sharing and therefore co-representation at least in humans is maintained as long as the relationship between the interaction partners is “good enough”, i.e. is not negative. This emphasizes the necessity for future studies with nonhuman primates to prioritize finding ways to also successfully test dyads at the lower end of social tolerance and relationship quality.
However, the requirement of strong social contrasts to detect an influence of social factors on co-representation may come from the fact that in humans, co-representation in joint Simon tasks is typically much weaker than co-representation observed in primates, and social effects may therefore be more difficult to detect. In animals, experimental studies on coordination behavior in the joint string-pulling task yield ambiguous results concerning the association between cooperation success and affiliation or closeness in rank between joint action partners. A positive relationship between cooperation success and social bond strength, closeness in rank, but further also inter-individual tolerance was found in wolves and ravens, both species that frequently cooperate in the wild (Massen et al. 2015; Asakawa-Haas et al. 2016; Marshall-Pescini et al. 2017; Dale et al. 2020). On the contrary, in barbary macaques, pairs with one adult and one low ranking juvenile were the most successful cooperators (Molesti and Majolo 2016) and in chimpanzees, cooperation was more successful between kin and individuals close in rank rather than between more strongly bonded individuals (Suchak et al. 2014). Therefore, depending on the species, inter-individual social tolerance might be just as, or more important than affiliation for cooperation success in joint action tasks. In our joint Simon task study in a group of 28 Tonkean macaques (22 individuals were ranked), mostly individuals of intermediate rank (five individuals were placed between rank 10 and 17 in the dominance hierarchy and only two individuals were higher ranked) participated. Consequently, joint action partners were mostly close in rank. In the group of 19 brown capuchins (14 individuals were ranked), the dyads showed almost exclusively the same pair-constellation over all sessions, which were those closest in rank and/or maternal kin. The alpha male did not tolerate the partner individual to simultaneously retrieve a rewarding food item and could therefore not be tested in the joint task. This is consistent with a potentially lower tolerance level for access to food in the most dominant male toward younger adult males observed in groups of capuchin monkeys in the wild (Janson 1985). The narrow range of rank differences and some preference for kin between partners in both monkey groups indeed suggest that all dyads that participated in the joint Simon task were highly tolerant.
The experimental results of animal studies on the joint string-pulling task indicate that in a joint action task in which cooperation success increases with SO integration and co-representation, also rather small differences in social tolerance and bond strength can be reflected in different levels of dyadic cooperation. Thus, also in the joint Simon task in primates, dyads with stronger social bonds may show stronger co-representation but simultaneously, they might make a greater effort to increase cooperation success which requires SO distinction in this specific task. A second explanation for the absence of a relationship between social factors and co-representation in the primates tested here may thus be that more affiliative or closer ranked dyads are more inclined to spontaneously co-represent their partner’s task and actions, but they are also better at suppressing it to maximize cooperation success. In a group of only highly socially tolerant individuals, this may result in the absence of any observable relationship in the joint Simon task. This is particularly likely if the ability to suppress (or activate) co-representation is not entirely determined by strong general cognitive factors (i.e., executive functions such as inhibitory control ability, or ToM).
Indeed, we found that general motor inhibitory control as measured with a detour-reaching task was linked neither to co-representation nor the ability to suppress it, within each of the three species but also when all species were analyzed together. Among the three species, the common marmosets showed the lowest level of motor inhibitory control, as expected from the fact that they have by far the smallest brains among the primates tested here (Deaner et al. 2007; MacLean et al. 2014; Burkart et al. 2017). Nevertheless, they were the least affected by automatic co-representation and showed the highest cooperation success.
Depending on the set-up and task administration, detour-reaching paradigms may rely more or less on additional skills such as causal reasoning, rule learning, attention or visual acuity, which may favor the use of detour task batteries over single tasks for species comparisons (Kabadayi et al. 2018). Variation in task-specific factors across species was controlled to some degree in our design by applying the same set-up (size of the barrier adjusted to body size) and procedure (in particular familiarization phase and number of trials) in all monkeys tested here, but individual differences in prior experience with cognitive tasks existed. We only measured one type of inhibitory control (detour-reaching) and different inhibition tasks likely measure different traits of this general cognitive skill (Audet and Lefebvre 2017). These include for instance motor inhibition (e.g., detour-reaching tasks, MacLean et al. 2014), self-control (e.g., delayed reward tasks, Evans et al. 2012) or the use of alternative behavioral strategies (e.g., reversal learning tasks, Manrique and Call 2015, or set-shifting tasks, Shnitko et al. 2017). Importantly, inhibitory control ability appears highly dependent on the context (e.g., in primates: Amici et al. 2018, in dogs: Brucks et al. 2017) and some species, despite having strong general inhibitory control, may show less behavioral flexibility in situations that require for instance a flexible switching of strategy adjusted to the social context (Amici et al. 2018). Correspondingly, highly flexible behavior in a social context (such as regulating or suppressing co-representation when necessary to optimize cooperation success) may be present in highly social species showing limited general inhibitory control, such as the common marmosets.
Thus, the primate data suggest that rather than primarily relying on general inhibitory control, the ability to suppress co-representation when necessary may be linked to specific processes of SO distinction in the motor domain (see Santiesteban et al. 2012), which may be trained when repeatedly engaging in cooperative interactions that require the balancing between SO integration vs. SO distinction. Highly social and interdependent species who rely on cooperation in their everyday life to facilitate joint infant care taking, such as humans and common marmosets (Hrdy 2009; Erb and Porter 2017), have recurrent opportunities to accumulate greater experience in joint activities and to become competent cooperators (e.g., in cooperative problem solving, Martin et al. 2021). This may advance the acquisition of abilities in social learning, such as behavior copying and imitation (Fletcher et al. 2012, in marmosets: Voelkl and Huber 2000, 2007), communication (Goldstein and Schwade 2008, in marmosets: Gultekin and Hage 2017; Takahashi et al. 2017), or coordination of attention and action (Bakeman and Adamson 1984; Moll et al. 2008). The necessity to coordinate complementary motor actions, such as handing over infants from one carrier to another or turn-taking during antiphonal calling (Snowdon 2001; Takahashi et al. 2013), and mutually exclusive activities among group members (e.g., feeding vs. vigilance, Brügger et al. 2022) arises frequently during shared infant care (Burkart et al. 2022; Hrdy and Burkart 2020). Such joint endeavors crucially require an optimal balancing between SO integration and SO distinction. Thus, during development and from an early age on, through interactions with their mothers, other caregivers (allomothers) and peers, infants may learn continuously when to merge and when to dissociate themselves from the other, while general cognitive skills (executive functions such as inhibitory control, and ToM) develop in parallel and may, at an advanced stage, come to support this process. Thus, a cooperative lifestyle may enhance the selective cognitive mechanisms to regulate and suppress co-representation when necessary through exposure to SO integration—distinction conflicts in joint action contexts and practice. This pathway of a supportive but not primary involvement of higher order cognition is consistent with the finding that stronger general inhibition and ToM skills were associated with weaker co-representation in a joint action task requiring complementary actions in 4- to 5-year-old children (Milward et al. 2017). Note that in humans, co-representation is hardly ever visible in actual errors but only in marginal delays in reaction times (commonly ranging between 10 and 30 ms; e.g., Sebanz et al. 2003; Kiernan et al. 2012; Pfister et al. 2014).
In the future, it is paramount to find ways to quantify both processes separately, the tendency to merge (i.e., spontaneously co-represent) as well as the ability to enhance SO distinction (i.e., suppress co-representation), and to measure for each of them separately how they are modulated by social factors. Joint Simon task studies in humans suggest that the influence of social factors increases with mutual dependency between co-actors (e.g., Ruys and Aarts 2010; Ford and Aberdein 2015). For such future investigations, it is thus desirable to find joint action tasks in which cooperation success increases with SO integration and co-representation without SO distinction to get rid of the confounding effect of the necessity to suppress automatic co-representation. Such paradigms may favor tasks with identical simultaneous task roles and include for instance synchronous movement (Kirschner and Tomasello 2009; Valdesolo et al. 2010), action imitation (Brass and Heyes 2005), or the mental coordination of decision-making (McClung et al. 2017). In fact, distinguishing between coordination tasks in which co-representation facilitates versus reduces cooperation success may as well help to explain weak or ambiguous results often reported in human co-representation in the joint Simon task (e.g., Sebanz et al. 2003; Pfister et al. 2014).
In sum, the emerging studies investigating the evolutionary origin of co-representation clearly show that co-representation is not unique to humans but most likely ancestral in primates, or at least haplorrhines. The flexibility to suppress co-representation when necessary among primates appears not contingent on strong general motor inhibitory control (and advanced higher order cognition) but seems rather strongest in those species who most routinely rely on, and thus accumulate greater experience in cooperation during their daily life, such as the cooperatively breeding common marmosets and humans. The advanced cognitive abilities of humans compared to primates were therefore not a precondition for the emergence of co-representation and cooperative flexibility during evolutionary times. Rather, it allowed our ancestors to deploy preexisting cooperative predispositions in ever more complex ways.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–266. https://doi.org/10.1163/156853974X00534
Amici F, Aureli F, Call J (2008) Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr Biol 18:1415–1419. https://doi.org/10.1016/j.cub.2008.08.020
Amici F, Call J, Watzek J, Brosnan S, Aureli F (2018) Social inhibition and behavioural flexibility when the context changes: a comparison across six primate species. Sci Rep 8:1–9. https://doi.org/10.1038/s41598-018-21496-6
Asakawa-Haas K, Schiestl M, Bugnyar T, Massen JJM (2016) Partner choice in raven (corvus corax) cooperation. PLoS ONE 11:1–15. https://doi.org/10.1371/journal.pone.0156962
Atmaca S, Sebanz N, Knoblich G (2011) The joint flanker effect: sharing tasks with real and imagined co-actors. Exp Brain Res 211:371–385. https://doi.org/10.1007/s00221-011-2709-9
Audet J-N, Lefebvre L (2017) What’s flexible in behavioral flexibility? Behav Ecol 28:943–947. https://doi.org/10.1093/beheco/arx007
Bakeman R, Adamson LB (1984) Coordinating attention to people and objects in mother-infant and peer-infant interaction. Child Dev 55:1278–1289. https://doi.org/10.2307/1129997
Ballesta S, Sadoughi B, Miss F, Whitehouse J, Aguenounon G, Meunier H (2021) Assessing the reliability of an automated method for measuring dominance hierarchy in non-human primates. Primates. https://doi.org/10.1007/s10329-021-00909-7
Bekkering H, De Bruijn ERA, Cuijpers RH, Newman-Norlund R, Van Schie HT, Meulenbroek R (2009) Joint action: neurocognitive mechanisms supporting human interaction. Top Cogn Sci 1:340–352. https://doi.org/10.1111/j.1756-8765.2009.01023.x
Bergstrom ML, Fedigan LM (2010) Dominance among female white-faced capuchin monkeys (Cebus capucinus): hierarchical linearity, nepotism, strength and stability. Behaviour 147:899–931. https://doi.org/10.1163/000579510X497283
Brass M, Heyes C (2005) Imitation: is cognitive neuroscience solving the correspondence problem? Trends Cogn Sci 9:489–495. https://doi.org/10.1016/j.tics.2005.08.007
Brass M, Ruby P, Spengler S (2009) Inhibition of imitative behaviour and social cognition. Philos Trans R Soc B Biol Sci 364:2359–2367. https://doi.org/10.1098/rstb.2009.0066
Brent LJN (2015) Friends of friends: are indirect connections in social networks important to animal behaviour? Anim Behav 103:211–222. https://doi.org/10.1016/j.anbehav.2015.01.020
Brucks D, Marshall-Pescini S, Wallis LJ, Huber L, Range F (2017) Measures of dogs’ inhibitory control abilities do not correlate across tasks. Front Psychol 8:849. https://doi.org/10.3389/fpsyg.2017.00849
Brügger RK, Willems EP, Burkart JM (in review) Looking out for each other: synchronization and turn taking in common marmoset vigilance. Anim Behav
Burkart JM, Hrdy SB, van Schaik CP (2009) Cooperative breeding and human cognitive evolution. Evol Anthropol 18:175–186. https://doi.org/10.1002/evan.20222
Burkart JM, Allon O, Amici F, Fichtel C, Finkenwirth C, Heschl A, Huber J, Isler K, Kosonen ZK, Martins E, Meulman EJ, Richiger R, Rueth K, Spillmann B, Wiesendanger S, van Schaik CP (2014) The evolutionary origin of human hyper-cooperation. Nat Commun 5:4747. https://doi.org/10.1038/ncomms5747
Burkart JM, Schubiger MN, van Schaik CP (2017) The evolution of general intelligence. Behav Brain Sci 40:e195. https://doi.org/10.1017/S0140525X16000959
Burkart JM, Adriaense JEC, Brügger RK, Miss FM, Wierucka K, van Schaik CP (2022) A convergent interaction engine: vocal communication among marmoset monkeys. Philos Trans R Soc B 2022:1–19
Burns P, Riggs KJ, Beck SR (2012) Executive control and the experience of regret. J Exp Child Psychol 111:501–515. https://doi.org/10.1016/j.jecp.2011.10.003
Butterfill S (2012) Joint action and development. Philos Q 62:23–47. https://doi.org/10.1111/j.1467-9213.2011.00005.x
Cheney DL, Silk JB, Seyfarth RM (2016) Network connections, dyadic bonds and fitness in wild female baboons. R Soc Open Sci. https://doi.org/10.1098/rsos.160255
Clark HH (2006) Social actions, social commitments. In: Enfield NJ, Levinson SC (eds) Roots of human sociality: culture, cognition and interaction. Berg, Oxford, pp 126–150
Constable MD, McEwen ES, Knoblich G, Call J (2021) Do chimpanzees represent the actions of a co-ordination partner? In: Proceedings of the annual meeting of the cognitive science society, p 43. https://escholarship.org/uc/item/11k4v28v
Dale R, Marshall-Pescini S, Range F (2020) What matters for cooperation? The importance of social relationship over cognition. Sci Rep. https://doi.org/10.1038/s41598-020-68734-4
de Hamilton AFC (2021) Hyperscanning: beyond the hype. Neuron 109:404–407. https://doi.org/10.1016/j.neuron.2020.11.008
de Waal FBM, Suchak M (2010) Prosocial primates: Selfish and unselfish motivations. Philos Trans R Soc B Biol Sci 365:2711–2722. https://doi.org/10.1098/rstb.2010.0119
de Oliveira Terceiro FE, de Arruda MF, van Schaik CP, Araújo A, Burkart JM (2021) Higher social tolerance in wild versus captive common marmosets: the role of interdependence. Sci Rep 11:1–10. https://doi.org/10.1038/s41598-020-80632-3
de Vries H, Stevens JMG, Vervaecke H (2006) Measuring and testing the steepness of dominance hierarchies. Anim Behav 71:585–592. https://doi.org/10.1016/j.anbehav.2005.05.015
Deaner RO, Isler K, Burkart JM, van Schaik C (2007) Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav Evol 70:115–124. https://doi.org/10.1159/000102973
Decety J, Sommerville JA (2003) Shared representations between self and other: a social cognitive neuroscience view. Trends Cogn Sci 7:527–533. https://doi.org/10.1016/j.tics.2003.10.004
Djalovski A, Dumas G, Kinreich S, Feldman R (2021) Human attachments shape interbrain synchrony toward efficient performance of social goals. Neuroimage 226:117600. https://doi.org/10.1016/j.neuroimage.2020.117600
Erb WM, Porter LM (2017) Mother’s little helpers: What we know (and don’t know) about cooperative infant care in callitrichines. Evol Anthropol 26:25–37. https://doi.org/10.1002/evan.21516
Evans TA, Perdue BM, Parrish AE, Menzel EC, Brosnan SF, Beran MJ (2012) How is chimpanzee self-control influenced by social setting? Sci (cairo) 2012:1–9. https://doi.org/10.6064/2012/654094
Fizet J, Rimele A, Pebayle T, Cassel JC, Kelche C, Meunier H (2017) An autonomous, automated and mobile device to concurrently assess several cognitive functions in group-living non-human primates. Neurobiol Learn Mem 145:45–58. https://doi.org/10.1016/j.nlm.2017.07.013
Fletcher GE, Warneken F, Tomasello M (2012) Differences in cognitive processes underlying the collaborative activities of children and chimpanzees. Cogn Dev 27:136–153. https://doi.org/10.1016/j.cogdev.2012.02.003
Ford RM, Aberdein B (2015) Exploring social influences on the joint Simon task: empathy and friendship. Front Psychol 6:962. https://doi.org/10.3389/fpsyg.2015.00962
Fujii K, Jin J, Vandeleest J, Shev A, Beisner B, McCowan B, Fushing H (2019) Perc: using percolation and conductance to find information flow certainty in a direct network. In: R Packag. version 0.1.3. https//cran.r-project.org/package=Perc
Gallese V, Goldman A (1998) Mirror neurons and the simulation theory of mind-reading. Trends Cogn Sci 2:493–501. https://doi.org/10.1016/S1364-6613(98)01262-5
Gerstadt CL, Hong YJ, Diamond A (1994) The relationship between cognition and action: performance of children 3 1/2 - 7 years old on a Stroop-like day-night test. Cognition 53:129–153. https://doi.org/10.1016/0010-0277(94)90068-X
Gokcekus SY (2020) Behavioral flexibility, curiosity, and cooperative breeding: dealing with complex concepts and paradigms. Thesis, Durham University. http://etheses.dur.ac.uk/13424/
Goldstein MH, Schwade JA (2008) Social feedback to infants’ babbling facilitates rapid phonological learning. Psychol Sci 19:515–523. https://doi.org/10.1111/j.1467-9280.2008.02117.x
Guerreiro Martins EM, Antonio AC, Finkenwirth C, Griesser M, Burkart JM (2019) Food sharing patterns in three species of callitrichid monkeys (Callithrix jacchus, Leontopithecus chrysomelas, Saguinus midas): individual and species differences. J Comp Psychol 133:474–487. https://doi.org/10.1037/com0000169
Gultekin YB, Hage SR (2017) Limiting parental feedback disrupts vocal development in marmoset monkeys. Nat Commun 8:14046. https://doi.org/10.1038/ncomms14046
Gvirts HZ, Perlmutter R (2020) What guides us to neurally and behaviorally align with anyone specific? A neurobiological model based on fNIRS hyperscanning studies. Neurosci 26:108–116. https://doi.org/10.1177/1073858419861912
Heesen R, Bangerter A, Zuberbühler K, Iglesias K, Neumann C, Pajot A, Perrenoud L, Guéry J-P, Rossano F, Genty E (2021) Assessing joint commitment as a process in great apes. iScience 24:102872. https://doi.org/10.1016/j.isci.2021.102872
Hommel B, Müsseler J, Aschersleben G, Prinz W (2001) The theory of event coding (TEC): a framework for perception and action planning. Behav Brain Sci 24:849–878. https://doi.org/10.1017/S0140525X01000103
Hommel B, Colzato LS, Van Den Wildenberg WPM (2009) How social are task representations? Psychol Sci 20:794–798. https://doi.org/10.1111/j.1467-9280.2009.02367.x
Hrdy SB (2009) Mothers & others: the evolutionary origins of mutual understanding. Harvard University Press, Cambridge
Hrdy SB, Burkart JM (2020) The emergence of emotionally modern humans: implications for language and learning. Philos Trans R Soc B Biol Sci 375:15–18. https://doi.org/10.1098/rstb.2019.0499
Iani C, Anelli F, Nicoletti R, Arcuri L, Rubichi S (2011) The role of group membership on the modulation of joint action. Exp Brain Res 211:439–445. https://doi.org/10.1007/s00221-011-2651-x
Iani C, Anelli F, Nicoletti R, Rubichi S (2014) The carry-over effect of competition in task-sharing: evidence from the joint Simon task. PLoS ONE. https://doi.org/10.1371/journal.pone.0097991
Janson C (1985) Aggresive competition and individual food consumption in wild brown capuchin monkeys (Cebus apella). Behav Ecol Sociobiol 18:125–138. https://doi.org/10.1007/BF00299041
Kabadayi C, Bobrowicz K, Osvath M (2018) The detour paradigm in animal cognition. Anim Cogn 21:21–35. https://doi.org/10.1007/s10071-017-1152-0
Keller PE, Novembre G, Hove MJ (2014) Rhythm in joint action: psychological and neurophysiological mechanisms for real-time interpersonal coordination. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2013.0394
Kiernan D, Ray M, Welsh TN (2012) Inverting the joint Simon effect by intention. Psychon Bull Rev 19:914–920. https://doi.org/10.3758/s13423-012-0283-1
Kirschner S, Tomasello M (2009) Joint drumming: social context facilitates synchronization in preschool children. J Exp Child Psychol 102:299–314. https://doi.org/10.1016/j.jecp.2008.07.005
Knoblich G, Butterfill S, Sebanz N (2011) Psychological research on joint action: theory and data. In: Ross B (ed) The psychology of learning and motivation. Academic Press, Cambridge, pp 59–101
Leca JB, Fornasieri I, Petit O (2002) Aggression and reconciliation in Cebus capucinus. Int J Primatol 23:979–998. https://doi.org/10.1023/A:1019641830918
MacLean EL, Hare B, Nunn CL, Addessi E, Amici F, Anderson RC, Aureli F, Baker JM, Bania AE, Barnard AM, Boogert NJ, Brannon EM, Bray EE, Bray J, Brent LJN, Burkart JM, Call J, Cantlon JF, Cheke LG et al (2014) The evolution of self-control. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1323533111
Manrique HM, Call J (2015) Age-dependent cognitive inflexibility in great apes. Anim Behav 102:1–6. https://doi.org/10.1016/j.anbehav.2015.01.002
Manrique HM, Völter CJ, Call J (2013) Repeated innovation in great apes. Anim Behav 85:195–202. https://doi.org/10.1016/j.anbehav.2012.10.026
Marshall-Pescini S, Schwarz JFL, Kostelnik I, Virányi Z, Range F (2017) Importance of a species’ socioecology: Wolves outperform dogs in a conspecific cooperation task. Proc Natl Acad Sci USA 114:11793–11798. https://doi.org/10.1073/pnas.1709027114
Martin JS, Koski SE, Bugnyar T, Jaeggi AV, Massen JJM (2021) Prosociality, social tolerance and partner choice facilitate mutually beneficial cooperation in common marmosets, Callithrix jacchus. Anim Behav 173:115–136. https://doi.org/10.1016/j.anbehav.2020.12.016
Massen JJM, Ritter C, Bugnyar T (2015) Tolerance and reward equity predict cooperation in ravens (Corvus corax). Sci Rep 5:1–11. https://doi.org/10.1038/srep15021
McClung JS, Jentzsch I, Reicher SD (2013) Group membership affects spontaneous mental representation: failure to represent the out-group in a joint action task. PLoS ONE 8:e79178. https://doi.org/10.1371/journal.pone.0079178
McClung JS, Placì S, Bangerter A, Clément F, Bshary R (2017) The language of cooperation: shared intentionality drives variation in helping as a function of group membership. Proc R Soc B Biol Sci. https://doi.org/10.1098/rspb.2017.1682
Melis AP, Hare B, Tomasello M (2006) Engineering cooperation in chimpanzees: tolerance constraints on cooperation. Anim Behav 72:275–286. https://doi.org/10.1016/j.anbehav.2005.09.018
Mendres KA, de Waal FBM (2000) Capuchins do cooperate: the advantage of an intuitive task. Anim Behav 60:523–529. https://doi.org/10.1006/anbe.2000.1512
Meyer M, van der Wel RPRD, Hunnius S (2016) Planning my actions to accommodate yours: joint action development during early childhood. Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2015.0371
Milward SJ, Kita S, Apperly IA (2017) Individual differences in children’s corepresentation of self and other in joint action. Child Dev 88:964–978. https://doi.org/10.1111/cdev.12693
Miss FM, Burkart JM (2018) Corepresentation during joint action in marmoset monkeys (Callithrix jacchus). Psychol Sci 29:984–995. https://doi.org/10.1177/0956797618772046
Miss FM, Meunier H, Burkart JM (2022) Primate origins of co-representation and cooperative flexibility: a comparative study with common marmosets (Callithrix jacchus), brown capuchins (Sapajus apella) and Tonkean macaques (Macaca tonkeana). J Comp Psychol. https://doi.org/10.1037/com0000315
Molesti S, Majolo B (2016) Cooperation in wild Barbary macaques: factors affecting free partner choice. Anim Cogn 19:133–146. https://doi.org/10.1007/s10071-015-0919-4
Moll H, Richter N, Carpenter M, Tomasello M (2008) Fourteen-month-olds know what “we” have shared in a special way. Infancy 13:90–101. https://doi.org/10.1080/15250000701779402
Müller BCN, Kühn S, Van Baaren RB, Dotsch R, Brass M, Dijksterhuis A (2011) Perspective taking eliminates differences in co-representation of out-group members’ actions. Exp Brain Res 211:423–428. https://doi.org/10.1007/s00221-011-2654-7
Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A (2011) Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Anim Behav 82:911–921. https://doi.org/10.1016/j.anbehav.2011.07.016
Newman-Norlund RD, Bosga J, Meulenbroek RGJ, Bekkering H (2008) Anatomical substrates of cooperative joint-action in a continuous motor task: virtual lifting and balancing. Neuroimage 41:169–177. https://doi.org/10.1016/j.neuroimage.2008.02.026
Novembre G, Sammler D, Keller PE (2016) Neural alpha oscillations index the balance between self-other integration and segregation in real-time joint action. Neuropsychologia 89:414–425. https://doi.org/10.1016/j.neuropsychologia.2016.07.027
Perry S, Rose L (1994) Begging and transfer of coati meat by white-faced capuchin monkeys, Cebus capucinus. Primates 35:409–415. https://doi.org/10.1007/BF02381950
Petit O, Desportes C, Thierry B (1992) Differential probability of “coproduction” in two species of macaque (Macaca tonkeana, M. mulatta). Ethology 90:107–120. https://doi.org/10.1111/j.1439-0310.1992.tb00825.x
Pfister R, Dolk T, Prinz W, Kunde W (2014) Joint response–effect compatibility. Psychon Bull Rev 21:817–822. https://doi.org/10.3758/s13423-013-0528-7
Prinz W (1997) Perception and action planning. Eur J Cogn Psychol 9:129–154. https://doi.org/10.1080/713752551
Ruissen MI, de Bruijn ERA (2015) Is it me or is it you? Behavioral and electrophysiological effects of oxytocin administration on self-other integration during joint task performance. Cortex 70:146–154. https://doi.org/10.1016/j.cortex.2015.04.017
Ruissen MI, de Bruijn ERA (2016) Competitive game play attenuates self-other integration during joint task performance. Front Psychol 7:274. https://doi.org/10.3389/fpsyg.2016.00274
Ruys KI, Aarts H (2010) When competition merges people’s behavior: Interdependency activates shared action representations. J Exp Soc Psychol 46:1130–1133. https://doi.org/10.1016/j.jesp.2010.05.016
Sahaï A, Desantis A, Grynszpan O, Pacherie E, Berberian B (2019) Action co-representation and the sense of agency during a joint Simon task: comparing human and machine co-agents. Conscious Cogn 67:44–55. https://doi.org/10.1016/j.concog.2018.11.008
Samson D, Apperly IA, Braithwaite JJ, Andrews BJ, Bodley Scott SE (2010) Seeing it their way: evidence for rapid and involuntary computation of what other people see. J Exp Psychol Hum Percept Perform 36:1255–1266. https://doi.org/10.1037/a0018729
Santiesteban I, White S, Cook J, Gilbert SJ, Heyes C, Bird G (2012) Training social cognition: from imitation to Theory of Mind. Cognition 122:228–235. https://doi.org/10.1016/j.cognition.2011.11.004
Schmitz L, Vesper C, Sebanz N, Knoblich G (2017) Co-representation of others’ task constraints in joint action. J Exp Psychol Hum Percept Perform 43:1480–1493. https://doi.org/10.1037/xhp0000403
Schubiger MN, Kissling A, Burkart JM (2019) Does opportunistic testing bias cognitive performance in primates? Learning from drop-outs. PLoS ONE 14:1–22. https://doi.org/10.1371/journal.pone.0213727
Sebanz N, Knoblich G (2021) Progress in joint-action research. Curr Dir Psychol Sci 30:138–143. https://doi.org/10.1177/0963721420984425
Sebanz N, Knoblich G, Prinz W (2003) Representing others’ actions: just like one’s own? Cognition 88:B11–B21. https://doi.org/10.1016/S0010-0277(03)00043-X
Sebanz N, Knoblich G, Prinz W (2005) How two share a task: corepresenting stimulus-response mappings. J Exp Psychol Hum Percept Perform 31:1234–1246. https://doi.org/10.1037/0096-1523.31.6.1234
Sebanz N, Bekkering H, Knoblich G (2006a) Joint action: bodies and minds moving together. Trends Cogn Sci 10:70–76. https://doi.org/10.1016/j.tics.2005.12.009
Sebanz N, Knoblich G, Prinz W, Wascher E (2006b) Twin peaks: an ERP study of action planning and control in coacting individuals. J Cogn Neurosci 18:859–870. https://doi.org/10.1162/jocn.2006.18.5.859
Sebanz N, Rebbechi D, Knoblich G, Prinz W, Frith CD (2007) Is it really my turn? An event-related fMRI study of task sharing. Soc Neurosci 2:81–95. https://doi.org/10.1080/17470910701237989
Shafaei R, Bahmani Z, Bahrami B, Vaziri-Pashkam M (2020) Effect of perceived interpersonal closeness on the joint Simon effect in adolescents and adults. Sci Rep 10:1–10. https://doi.org/10.1038/s41598-020-74859-3
Shnitko TA, Allen DC, Gonzales SW, Walter NAR, Grant KA (2017) Ranking cognitive flexibility in a group setting of rhesus monkeys with a set-shifting procedure. Front Behav Neurosci 11:55. https://doi.org/10.3389/fnbeh.2017.00055
Silk JB (2007) The adaptive value of sociality in mammalian groups. Philos Trans R Soc B Biol Sci 362:539–559. https://doi.org/10.1098/rstb.2006.1994
Silk J, Cheney D, Seyfarth R (2013) A practical guide to the study of social relationships. Evol Anthropol 22:213–225. https://doi.org/10.1002/evan.21367
Snowdon CT (2001) Social processes in communication and cognition in callitrichid monkeys: a review. Anim Cogn 4:247–257. https://doi.org/10.1007/s100710100094
Sommerville JA, Decety J (2006) Weaving the fabric of social interaction: articulating developmental psychology and cognitive neuroscience in the domain of motor cognition. Psychon Bull Rev 13:179–200. https://doi.org/10.3758/BF03193831
Southgate V (2020) Are infants altercentric? The other and the self in early social cognition. Psychol Rev 127:505–523. https://doi.org/10.1037/rev0000182
Spengler S, Brass M, Kühn S, Schütz-Bosbach S (2010) Minimizing motor mimicry by myself: self-focus enhances online action-control mechanisms during motor contagion. Conscious Cogn 19:98–106. https://doi.org/10.1016/j.concog.2009.12.014
Steinbeis N (2016) The role of self–other distinction in understanding others’ mental and emotional states: neurocognitive mechanisms in children and adults. Philos Trans R Soc B 371:20150074. https://doi.org/10.1098/rstb.2015.0074
Suchak M, Eppley TM, Campbell MW, de Waal FBM (2014) Ape duos and trios: spontaneous cooperation with free partner choice in chimpanzees. PeerJ 2014:1–19. https://doi.org/10.7717/peerj.417
Takahashi DY, Narayanan DZ, Ghazanfar AA (2013) Coupled oscillator dynamics of vocal turn-taking in monkeys. Curr Biol 23:2162–2168. https://doi.org/10.1016/j.cub.2013.09.005
Takahashi DY, Liao DA, Ghazanfar AA (2017) Vocal learning via social reinforcement by infant marmoset monkeys. Curr Biol 27:1844-1852.e6. https://doi.org/10.1016/j.cub.2017.05.004
Thierry B, Gauthier C, Peignot P (1990) Social grooming in Tonkean macaques (Macaca tonkeana). Int J Primatol 11:357–375. https://doi.org/10.1007/BF02193006
Thierry B, Anderson JR, Demaria C, Desportes C, Petit O (1994) Tonkean macaque behaviour from the perspective of the evolution of Sulawesi macaques. In: Roeder JJ, Thierry B, Anderson JR, Herrenschmidt N (eds) Current primatology, social development, learning and behaviour. Université Louis Pasteur, pp 103–117
Thierry B, Bynum EL, Baker S, Kinnaird MF, Matsumura S, Muroyama Y, O’Brien TG, Petit O, Watanabe K (2000) The social repertoire of Sulawesi macaques. Primate Res 16:203–226. https://doi.org/10.2354/psj.16.203
Tomasello M (2019) The role of roles in uniquely human cognition and sociality. J Theory Soc Behav 50:2–19. https://doi.org/10.1111/jtsb.12223
Tsai C-C, Kuo WJ, Jing JT, Hung DL, Tzeng OJL (2006) A common coding framework in self-other interaction: evidence from joint action task. Exp Brain Res 175:353–362. https://doi.org/10.1007/s00221-006-0557-9
Tsai C-C, Kuo W-J, Hung DL, Tzeng OJL (2008) Action co-representation is tuned to other humans. J Cogn Neurosci 20:2015–2024. https://doi.org/10.1162/jocn.2008.20144
Valdesolo P, Ouyang J, DeSteno D (2010) The rhythm of joint action: Synchrony promotes cooperative ability. J Exp Soc Psychol 46:693–695. https://doi.org/10.1016/j.jesp.2010.03.004
Vesper C, Butterfill S, Knoblich G, Sebanz N (2010) A minimal architecture for joint action. Neural Netw 23:998–1003. https://doi.org/10.1016/j.neunet.2010.06.002
Vesper C, van der Wel RPRD, Knoblich G, Sebanz N (2013) Are you ready to jump? Predictive mechanisms in interpersonal coordination. J Exp Psychol Hum Percept Perform 39:48–61. https://doi.org/10.1037/a0028066
Vesper C, Knoblich G, Sebanz N (2014) Our actions in my mind: Motor imagery of joint action. Neuropsychologia 55:115–121. https://doi.org/10.1016/j.neuropsychologia.2013.05.024
Vesper C, Abramova E, Bütepage J, Ciardo F, Crossey B, Effenberg A, Hristova D, Karlinsky A, McEllin L, Nijssen SRR, Schmitz L, Wahn B (2017) Joint action: mental representations, shared information and general mechanisms for coordinating with others. Front Psychol 7:2039. https://doi.org/10.3389/fpsyg.2016.02039
Vilette C, Bonnell T, Henzi P, Barrett L (2020) Comparing dominance hierarchy methods using a data-splitting approach with real-world data. Behav Ecol 31:1379–1390. https://doi.org/10.1093/beheco/araa095
Vlamings PHJM, Hare B, Call J (2010) Reaching around barriers: the performance of the great apes and 3–5-year-old children. Anim Cogn 13:273–285. https://doi.org/10.1007/s10071-009-0265-5
Voelkl B, Huber L (2000) True imitation in marmosets. Anim Behav 60:195–202. https://doi.org/10.1006/anbe.2000.1457
Voelkl B, Huber L (2007) Imitation as faithful copying of a novel technique in marmoset monkeys. PLoS ONE 2:e611. https://doi.org/10.1371/journal.pone.0000611
Acknowledgements
We thank all of the staff of the Primate Centre of Strasbourg University and Silabe, in particular the primate care taking staff for animal husbandry and the technician staff for support on construction of the experimental devices and set-up. We thank Marie Hirel for her initial help in learning animal ID’s, Katie Hallsten and Solène Lehman for their help during data collection, and Max Stoller for his help in video coding. We would like to thank Jamie Whitehouse for his help during the social network analyses, Erik Willems for general statistical advice, Carel van Schaik for valuable input on the study topic as well as the editor and reviewer for their helpful comments.
Funding
Open access funding provided by University of Zurich. The study was supported by the Swiss National Science Foundation (Grant no. 31003A_172979) granted to JMB, the NCCR Evolving Language, Swiss National Science Foundation (agreement no. 51NF40_180888) and the A. H. Schultz Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Experimental data collection was performed by FMM, behavioral data were collected by BS and FMM, and data analysis and interpretation was performed by FMM and JMB. The first draft of the manuscript was written by FMM and JMB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflicts of interest. The manuscript has not been published previously and is not under consideration for publication elsewhere.
Ethics approval
The study was conducted non-invasively. Data collection in France were performed according to the French legal requirements for the use of animals in research and complied with the EU Directive 2010/63/EU on the welfare of animals used for scientific purposes. Data collection in Switzerland was approved by the Kantonales Veterinäramt, License No. 223/16.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miss, F.M., Sadoughi, B., Meunier, H. et al. Individual differences in co-representation in three monkey species (Callithrix jacchus, Sapajus apella and Macaca tonkeana) in the joint Simon task: the role of social factors and inhibitory control. Anim Cogn 25, 1399–1415 (2022). https://doi.org/10.1007/s10071-022-01622-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-022-01622-8