Abstract
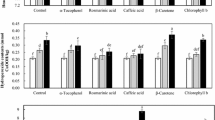
This study evaluated the pH effect on the lipid oxidation and polyphenols of the emulsions consisting of soybean oil, citric acid buffer (pH 2.6, 4.0, or 6.0), and peppermint (Mentha piperita) extract (400 mg/kg), with/without FeSO4. The emulsions in tightly-sealed bottles were placed at 25 °C in the dark, and lipid oxidation and polyphenol contents and composition were determined. The lipid oxidation was high in the emulsions at pH 4.0 in the absence of iron, however, iron addition made them more stable than the emulsions at pH 2.6 or 6.0. Total polyphenols were remained at the lowest content during oxidation in the emulsions at pH 4.0, and iron reduced and decelerated polyphenol degradation. The results strongly suggest that polyphenols contributed to decreased lipid oxidation of the emulsion via radical scavenging and iron-chelation, and rosmarinic acid along with catechin, caffeic acid, and luteolin were key polyphenols as radical scavengers in the extract.



Similar content being viewed by others

References
Abdalla AE, Roozen JP. Effect of plant extracts on the oxidative stability of sunflower oil and emulsion. Food Chem. 64: 323–329 (1999)
Kim J, Choe E. Effects of selected herb extracts on iron-catalyzed lipid oxidation in soybean oil-in-water emulsion. Food Sci. Biotechnol. 25: 1017–1022 (2016)
Quideau S. Why bother with polyphenols? (2011). Groupe Polyphenols. http://www.groupepolyphenols.com/the-society/why-bother-with-polyphenols/accessed(retrieved) on July 01, 2017.
Haslam E, Cai Y. Plant polyphenols (vegetable tannins): Gallic acid metabolism. Nat. Prod. Rep. 11: 41–66 (1994)
Drynan JW, Clifford MN, Obuchowicz J, Kuhnert N. The chemistry of low molecular weight black tea polyphenols. Nat. Prod. Rep. 27: 417–462 (2010)
Hider RC, Liu ZD, Khodr HH. Metal chelation of polyphenols. Methods Enzymol. 335: 190–203 (2001)
Zorić Z, Markić J, Pedisić S, Bučević-Popović V, Generalić-Mekinić I, Grebenar K, Kulišić-Bilušić T. Stability of rosmarinic acid in aqueous extracts from different Lamiaceae species after in vitro digestion with human gastrointestinal enzymes. Food Technol. Biotechnol. 54: 97–102 (2016)
Dinis PC, Falé PL, Amorim Madeira PJ, Floręncio MH, Serralheiro ML. Acetylcholinesterase inhibition activity after in vitro gastrointestinal digestion of infusions of Mentha species. European J. Med. Plants. 3: 381–393 (2013)
Arabshahi DS, Devi DV, Urooj A. Evaluation of antioxidant activity of some plant extracts and their heat, pH and storage stability. Food Chem. 100: 1100–1105 (2007)
Paiva-Martins F, Gordon MH. Effects of pH and ferric ions on the antioxidant activity of olive polyphenols in oil-in-water emulsions. J. Am. Oil Chem. Soc. 79: 571–576 (2002)
AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society. 4th ed. Method Cd 18-90. AOCS Press, Champaign, IL, USA (2006)
Guedon DJ, Pasquier BP. Analysis and distribution of flavonoid glycosides and rosmarinic acid in 40 Mentha piperita clones. J. Agric. Food Chem. 42: 679–684 (1994)
Hadjmohammadi M, Karimiyan H, Sharifi V. Hollow fibre-based liquid phase microextraction combined with high-performance liquid chromatography for the analysis of flavonoids in Echinophora platyloba DC and Mentha piperita. Food Chem. 141: 731–735 (2013)
Dent M, Dragovic-Uzelac V, Penic M, Brncic M, Bosiljkov T, Levaj B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 51: 84–91 (2013)
Chen J, Sun H, Wang Y, Wang Sn, Tao X, Sun A. Stability of apple polyphenols as a function of temperature and pH. Intern. J. Food Prop. 17: 1742–1749 (2014)
Huang S-W, Frankel EN, Schwarz K, German JB. Effect of pH on antioxidant activity of α-tocopherol and trolox in oil-in-water emulsions. J. Agric. Food Chem. 44: 2496–2502 (1996)
Li N, Taylor LS, Ferruzzi MG, Mauer LJ. Kinetic study of catechin stability: Effects of pH, concentration, and temperature. J. Agric. Food Chem. 60: 12531–12539 (2012)
García-Casal MN, Layrisse M. The effect of change in pH on the solubility of iron bis-glycinate chelate and other iron compounds. Arch. Latinoam. Nutr. 51(Suppl 1): 35–36 (2001)
Morgan B, Lahav O. The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution – basic principles and a simple heuristic description. Chemosphere 68: 2080–2084 (2007)
Choe E. Min DB. Mechanisms of antioxidants in the oxidation of foods. Comp. Rev. Food Sci. Food Saf. 8: 345–358 (2009)
Psotová J, Lasovsky J, Vicar J. Metal-chelating properties, electrochemical behavior, scavenging and cytoprotective activities of six natural phenolics. Biomed. Papers. 147: 147–153 (2003)
Brewer MS. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Comp. Rev. Food Sci. Food Saf. 10: 221–247 (2011)
Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 53: 75–100 (2009)
Perron NR, Wang HC, DeGuire, SN, Jenkins M, Lawson M, Brumaghim JL. Kinetics of iron oxidation upon polyphenol binding. Dalton Trans. 39: 9982–9987 (2010)
Kim J, Choe E. Improvement of the lipid oxidative stability of soybean oil-in water emulsion by addition of daraesoon (shoot of Actinidia arguta) and samnamul (shoot of Aruncus dioicus) extract. Food Sci. Biotechnol. 26: 113–119 (2017)
Sroka Z, Fecka I, Cisowski W. Antiradical and anti-H2O2 properties of polyphenolic compounds from an aqueous peppermint extract. Z. Naturforsch C. 60: 826–832 (2005)
Panya A, Kittipongpittaya K, Laguerre M, Bayrasy C, Lecomte J, Villeneuve P, McClements DJ, Decker EA. Interactions between α-tocopherol and rosmarinic acid and its alkyl esters in emulsions: synergistic, additive, or antagonistic effect? J. Agric. Food Chem. 60: 10320–10330 (2012)
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1C1A2A01053245).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kim, J., Choe, E. Effect of the pH on the lipid oxidation and polyphenols of soybean oil-in-water emulsion with added peppermint (Mentha piperita) extract in the presence and absence of iron. Food Sci Biotechnol 27, 1285–1292 (2018). https://doi.org/10.1007/s10068-018-0324-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-018-0324-2