Abstract
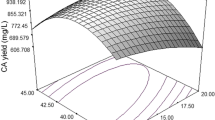
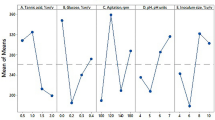
The nutritional requirements for antimicrobial activity of Streptomyces rimosus AG-P1441 were optimized using statistically-based experimental designs at a flask level. Based on a one-factor-at-a-time (OFAT) approach, glucose, corn starch and soybean meal were identified as the carbon and nitrogen sources having a significant effect on antimicrobial productivity. As a result of investigating the effect of glucose concentration, the highest antimicrobial activity was observed at 3% concentration. Response surface methodology (RSM) was then applied to optimize the growth medium components (corn starch, soybean meal, MgCl2 and glutamate). Antimicrobial productivity increased sharply when the medium consisted of 3% glucose, 3.5% corn starch, 2.5% soybean meal, 1.2 mM MgCl2 and 5.9 mM glutamate. The fermentation using optimized culture medium in a 5-L bioreactor allowed a significant increase in antimicrobial activity, evaluated by the paper disc assay, revealed a 29 mm inhibition zone diameter against Phytophthora capsici.



Similar content being viewed by others
References
Hausbeck MK, Lamour KH. Phytophthora capsici on vegetable crops: research progress and management challenges. Plant Dis. 88: 1292–1300 (2014)
Chen YY, Chen PC, Tsay TT. The biocontrol efficacy and antibiotic activity of Streptomyces plicatus on the oomycete Phytophthora capsici. Biol. Control. 98: 34–42 (2016)
Gavino PD, Smart CD, Sandrock RW, Miller JS, Hamm PB, Lee TY, Davis RM, Fry WE. Implications of sexual reproduction for Phytophthora infestans in the United States: generation of an aggressive lineage. Plant Dis. 84: 731–735 (2000)
Zohara F, Akanda AM, Paul NC, Rahman M, Islam T. Inhibitory effects of Pseudomonas spp. on plant pathogen Phytophthora capsici in vitro and in planta. Biocatal. Agric. Biotechnol. 5: 69–77 (2016)
Lee HB, Kim Y, Kim JC, Choi GJ, Park SH, Kim CJ, Jung HS. Activity of some aminoglycoside antibiotics against true fungi, Phytophthora and Pythium species. J. Appl. Microbiol. 99: 836–843 (2005)
Rothrock CS and Gottlieb D. Importance of antibiotic production in antagonism of Streptomyces species to two soil-borne plant pathogens. J. Antibiot. 34: 830–835 (1981)
Crawford DL, Linch JM, Whipps JM and Ousley MA. Isolation and characterization of actinomycete antagonists of a fungal root pathogen. Appl. Environ. Microbiol. 59: 3899–3905 (1993)
Mari M, Guizzardi M and Pratella GC. Biological control of gray mold in pears by antagonistic bacteria. Biol. Control. 7: 30–37 (1996)
Georgakopoulos DG, Fiddaman P, Leifert C and Malathrakis NE. Biological control of cucumber and sugar beet damping off caused by Pythium ultimum with bacterial and fungal antagonists. J. Appl. Microbiol. 92: 1078–1086 (2002)
Kim CJ, Park DJ, Lee JC, Ju YJ, Yoon BS. Development of biocontrol agent from bioactive compounds of microbial origin. Annual report of Rural Development Administration. (2013)
Rathi P, Goswami VK, Sahai V, Gupta R. Statistical medium optimization and production of a hyperthermostable lipase from Burkholderia cepacia in a bioreactor. J. Appl. Microbiol. 93(6): 930–936 (2002)
Grove and Randall. Assay methods of antibiotics medical encyclopedia. Inc. New York, NY, USA. (1955)
Singh AK, Mehta G, Chhatpar HS. Optimization of medium constituents for improved chitinase production by Paenibacillus sp. D1 using statistical approach. Lett. Appl. Microbiol. 49(6): 708–714 (2009)
Plackett RL and Burman JP. The design of optimum multifactorial experiments. Biometrika. 33(4): 305–325 (1946)
Box G, Hunter W, Hunter J. Statistics for experiments. Wiley, New York, USA. (1958)
Singh V, Haque S, Niwas R, Srivastava A, Pasupuleti M, Tripathi CK. Strategies for Fermentation Medium Optimization: An In-Depth Review. Front. Microbiol. Jan 6;7:2087 (2017)
Sánchez S, Chávez A, Forero A, García-Huante Y, Romero A, Sánchez M, Rocha D, Sánchez B, Avalos M, Guzmán-Trampe S, Rodríguez-Sanoja R, Langley E, Ruiz B. Carbon source regulation of antibiotic production. J. Antibiot. 63: 442–459 (2010)
Aharonowitz, Y. Nitrogen metabolite regulation of antibiotic biosynthesis. Ann. Rev. Microbiol. 34: 209–233 (1980)
Porter MA, Jones AM. Variability in soy flour composition. J. Am. Oil Chem. Soc. 80(6): 557–562 (2003)
Schrader KK, Blevins WT. Effects of carbon source, phosphorus concentration, and several micronutrients on biomass and geosmin production by Streptomyces halstedii. J. Ind. Microbiol. Biotechnol. 26(4): 241–247 (2001)
Natsume M, Kamo Y, Hirayama M, Adachi T. Isolation and characterization of alginate-derived oligosaccharides with roof growth-promoting activities. Carbohydr. Res. 258: 187–197 (1994)
Okba AK, Ogata T, Matsubara H, Matsuo S, Doi K, Ogata S. Effects of bacitracin and excess Mg2+ on submerged mycelial growth of Streptomyces azureus. J. Ferment. Bioeng. 86: 28–33 (1998)
Lubbe C, Jensen SE, Demain AL. Prevention of phosphate inhibition of cephalosporin synthetases by ferrous ion. FEMS Microbiol. Lett. 25: 75–79 (1984)
Raza W, Yang XM, Wu HS, Huang QW, Xu YC, Shen QR. Evaluation of metal ions (Zn2+, Fe3+, Mg2+) effect on production of fusaricidin-type antifungal compounds by Paenibacillus polymyxa SQR-2. Bioresour. Technol. 101: 9264–9271 (2010)
Mahmood M. Trace elements for growth and bulbiformin production by Bacillus subtilis. J. Appl. Bacteriol. 35: 1–5 (1972)
Liu CM, McDaniel LE, Schaffner CP. Factors affecting the production of Candicidin. Antimicrob. Agents. Chemother. 7: 196–202 (1975)
Voelker F, Altaba S. Nitrogen source governs the patterns of growth and pristinamycin production in ‘Streptomyces pristinaespiralis’ Microbiology. 147: 2447–2459 (2001)
Sissi C, Palumbo M. Effects of magnesium and related divalent metal ions in topoisomerase structure and function. Nucleic Acids Res. Feb;37(3): 702–711 (2009)
Cowan JA. Introduction to the biological chemistry of magnesium ion. The biological chemistry of magnesium. VCH. New York, 1–23 (1995)
Box, GEP, Wilson, KB. On the experimental attainment of optimum conditions. J. Roy. Stat. Soc. (Ser. B) 13: 1–45 (1951)
Rajeswari P, Jose PA, Amiya R, Jebakumar SR. Characterization of saltern based Streptomyces sp. and statistical media optimization for its improved antibacterial activity. Front. Microbiol. 5: 753 (2015)
Gao H, Liu M, Liu J, Dai H, Zhou X, Liu X, Zhuo Y, Zhang W, Zhang L. Medium optimization for the production of avermectin B1a by Streptomyces avermitilis 14-12A using response surface methodology. Bioresour. Technol. 100: 4012–4016 (2009)
Vineeta Singh, C.K.M. Tripathi. Production and statistical optimization of a novel olivanic acid by Streptomyces olivaceus MTCC 6820. Process biochem. 43: 1313–1317 (2008)
Chen XC, Bai JX, Cao JM, Li ZJ, Xiong J, Zhang L, Hong Y, Ying HJ. Medium optimization for the production of cyclic adenosine 3′,5′-monophosphate by Microbacterium sp. no. 205 using response surface methodology. Bioresour. Technol. 100: 919–924 (2009)
Acknowledgements
This research was supported by a Grant (10045326) from the R&D Program of MOTIE/KEIT of Republic of Korea, the KRIBB Research Initiative Program, Republic of Korea and a Grant (NRF-2013M3A9A5076601) from a study on the strategies of improving the value of microbial resources funded by Ministry of Science, ICT and Future Planning of the Korea Government.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ju, Y., Son, KH., Jin, C. et al. Statistical optimization of culture medium for improved production of antimicrobial compound by Streptomyces rimosus AG-P1441. Food Sci Biotechnol 27, 581–590 (2018). https://doi.org/10.1007/s10068-017-0257-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0257-1