Abstract
Clinical practice guidelines can assist rheumatologists in the proper prescription of newer treatment for rheumatoid arthritis (RA). The objective of this paper is to report on the recommendations for the management of patients with RA in the Eastern Mediterranean region. We adapted the 2015 American College of Rheumatology guidelines in two separate waves. We used the adolopment methodology, and followed the 18 steps of the “Guidelines 2.0” comprehensive checklist for guideline development. For each question, we updated the original guidelines’ evidence synthesis, and we developed an Evidence Profile (EP) and an Evidence to Decision (EtD) table. In the first wave, we adoloped eight out of the 15 original questions on early RA. The strength changed for five of these recommendations from strong to conditional, due to one or more of the following factors: cost, impact on health equities, the balance of benefits, and harms and acceptability. In the second wave, we adoloped eight out of the original 44 questions on established RA. The strength changed for two of these recommendations from strong to conditional, in both cases due to cost, impact on health equities, balance of benefits and harms, and acceptability. The panel also developed a good practice recommendation. We successfully adoloped 16 recommendations for the management of early and established RA in the Eastern Mediterranean region. The process proved feasible and sensitive to contextual factors.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is an inflammatory joint disease that causes pain, swelling, and disability in advanced stages [1,2,3,4]. It is ranked 42nd following malaria in contributing to global disability [5]. Its prevalence is 3/1000, with women affected more than men [5, 6]. In the Middle-East, the prevalence of RA is around 0.16% [5].
The management of RA aims to decrease symptoms, improve quality of life, and prevent disease progression [7]. The choice of treatment depends upon disease activity, prognostic factors, and patients’ responsiveness to previous lines of treatment [8, 9] with several effective options available. Guidelines are helpful to guide physicians, patients, and payers to choose the appropriate management plan.
De novo development of guidelines is time consuming and requires large financial and human resources. Also, adopting published guidelines developed for another setting is not desirable, given the importance of contextual factors in developing recommendations. Adaptation of guidelines addresses the above challenges, as it requires less resources and time and considers contextual factors in the process of adaptation.
The objective of this manuscript is to report on the adapted recommendations for the management of RA in the EMR (See Supplementary material for the Executive Summary).
Methodology
We adapted these guidelines in two waves in 2016 and 2017 using the GRADE approach.
We have previously reported the methodology used for this project [10] in relation to the following: (1) groups and roles; (2) selecting guideline topics; (3) identifying and training guideline panelists; (4) prioritizing questions and outcomes; (5) identifying, updating, or conducting systematic reviews; (6) preparing GRADE evidence tables and EtD frameworks; (7) formulating and grading strength of recommendations; (8) using the GRADEpro-GDT software (www.gradepro.org).
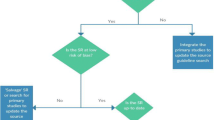
Briefly, we followed the adolopment methodology [11], an efficient model for guideline production based on adoption, adaptation, and development of recommendations utilizing the GRADE Evidence to Decision (EtD) frameworks [12, 13]. To implement this methodology, we followed the 18 steps of the GIN-McMaster guideline development tool (hei.mcmaster.ca/guidecheck.html) based on the “Guidelines 2.0” comprehensive checklist for guideline development [14]. The target end-users of the recommendations were rheumatologists in the EMR managing patients with RA.
The adaptation was based on the “2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis,” as the source guidelines [15]. Through a formal prioritization process, we selected a limited number of recommendations to adapt in each of the two waves. We also used a formal process to select patient important outcomes relevant to the selected questions.
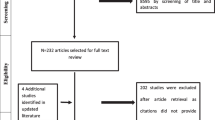
For each question, the guideline coordination team updated the 2014 evidence review conducted for the source guidelines. This included searches for both systematic reviews and primary studies relevant to the selected questions (see Appendix d for search strategies). We assessed identified systematic reviews for relevance (directness), quality (risk of bias) using AMSTAR, and up-to-datedness. When necessary, we updated the original meta-analysis. We ran additional searches relating to values and preferences and resource use, the latter being restricted to the EMR.
The guideline coordination team then developed an Evidence Profile (EP) [16, 17] and an EtD table following the GRADE approach [12, 13, 18]. For each of the two waves, the guideline panel met in person to discuss and adapt the recommendations.
The panel rated the certainty of evidence supporting each recommendation according to the GRADE methodology, as “high,” “moderate,” “low,” or “very low” [19, 20] (Table 1). The panel graded the strength of each recommendation as either strong or conditional (also known as or called weak) [21] (Table 2). The factors considered when grading the strength of recommendation were as follows: priority of the problem, benefits and harms of the option, certainty of the evidence, values and preferences, resource use, feasibility, acceptability, and equity.
During their second meeting, the panelists developed a good practice statement, which represents a recommendation that “guideline panels feel is important but that, in the judgment of the GRADE working group, is not appropriate for formal ratings of quality of evidence” [20].
Results
Through a formal prioritization process, we selected eight out of the 15 original questions on early RA to adapt in wave 1 (Table 3), and eight out of the original 44 questions on established RA to adapt in wave 2 (Table 4). In addition, the panel developed one good practice statement [20].
Good practice statement
In patients with RA, the panel recommends that healthcare practitioners provide patients with the needed education about the nature of disease, its progression, the various types of medications, and their expected benefits and harms. (See Table 5 for the good practice statement checklist) [20].
Early RA recommendations
Results of the general search for wave 1
The search for systematic reviews of effectiveness yielded 772 papers published after the date of the search conducted for the ACR guidelines. Only two were relevant to the project [22, 23]. The systematic search for primary studies of effectiveness yielded 2051 papers, out of which five were eligible [24,25,26,27,28] (Appendix e).
We identified 16 papers addressing patients’ values and preferences. None were specific to the EMR. Based on the review of these papers, the panelists judged that there is probably no uncertainty or variability in how much people value the main outcomes: pain, function, and avoiding adverse effects, across all guideline questions. This judgment was later reflected in the EtD tables (Appendix f). We could not find any studies on resource use relevant to the EMR.
Wave 1 recommendations (early RA)
Question 1
In patients with early RA, should we use treat-to-target strategy versus a non-targeted approach?
Health effects
The panelists judged that the balance between benefits and harms probably favors the treat-to-target approach. This judgment reflected a higher value placed on possible efficacy relative to a slight increase in side effects.
Contextual factors
The panelists judged the intervention to be associated with moderate cost, probably acceptable for most stakeholders, and probably feasible. However, they were unclear about its effects on equity.
Recommendation 1
In patients with early RA, the panel suggests using a treat-to-target strategy versus a non-targeted approach (conditional recommendation, low certainty evidence).
Conditions:
-
Consider the potential burden associated with extra costs and time for patients and physicians
-
Educate the patient on what treat to target intervention entails
Question 2
In patients with early RA with moderate or high disease activity, who are DMARD-naive, should we use combination double DMARD therapy versus mono-DMARD therapy?
Health effects
The panel judged that the balance of benefits and harms was not in favor of either double-DMARD therapy or mono-DMARD therapy.
Contextual factors
The panelists judged the intervention to be associated with moderate cost and increased health inequities. They found it to be probably feasible to implement but not acceptable by key stakeholders.
Recommendation 2
In patients with early RA with moderate or high disease activity, who are DMARD-naïve, the panel suggests not using combination double-DMARD therapy versus mono-DMARD (conditional recommendation, low certainty evidence).
Condition:
-
Consider patient’s perspective related to the number of pills prescribed.
Question 3
In patients with early RA with moderate or high disease activity, who are DMARD-naive, should we use combination triple traditional DMARD therapy versus mono-DMARD therapy?
Health effects
The panelists decided that the balance between desirable and undesirable effects probably favors treating patients with early RA with DMARD monotherapy rather than triple DMARD therapy.
Contextual factors
The panelists judged the intervention to be associated with moderate cost and increased health inequities. They also judged it to be probably feasible to implement in the EMR but not acceptable by key stakeholders.
Recommendation 3
In patients with early RA with moderate or high disease activity, who are DMARD-naïve, the panel suggests not using combination triple traditional DMARD therapy versus mono-DMARD therapy (Conditional recommendation, Low certainty of evidence).
Condition:
-
Consider patient’s perspective related to the number of pills prescribed.
Question 4
In patients with early RA with moderate or high disease activity, should we add versus not add long-term low-dose glucocorticoid therapy to traditional DMARDs?
Health effects
The panel agreed that the balance between desirable and undesirable effect is variable but probably in favor of adding low-dose glucocorticoids to traditional treatment.
Contextual factors
The panel members were not certain about the magnitude of the resource requirements for glucocorticoids. They judged the intervention to probably increase health equities, to be feasible to implement, and to be probably acceptable by key stakeholders in the condition of offering low-dose glucocorticoids for a short period of time.
Recommendation 4
In patients with early RA with moderate or high disease activity, the panel suggests adding over not adding long-term low-dose glucocorticoid therapy to traditional DMARDs (conditional recommendation, very low certainty of evidence).
Conditions:
-
Prescribe the lowest possible dose of glucocorticoids for the shortest possible period of time.
-
Monitor patients regularly.
-
Continuously assess for the development of co-morbidities (e.g., osteoporosis, hypertension, diabetes mellitus, and dyslipidemia).
Question 5
In patients with early RA with moderate or high disease activity, who have failed traditional DMARD therapy, should we use TNFi monotherapy versus triple DMARD therapy?
Health effects
The panel judged that the balance between desirable and undesirable effect does not favor either TNFI monotherapy or triple DMARD therapy. These judgments were supported by the indirect evidence brought forward during the panel meeting.
Contextual factors
The panel members judged the intervention to be associated with large cost and a reduction in health equities. They deemed it as probably acceptable and probably feasible to key stakeholders.
Recommendation 5
In patients with early RA with moderate or high disease activity, who have failed traditional DMARD therapy, the panel suggests triple DMARD therapy over TNFi monotherapy (conditional recommendation, low certainty of evidence).
Conditions:
-
Consider TNFi therapy in patients who do not mind or prefer injections over multiple pill intake and when resources are available.
-
Consider the additional possible harms of TNFi in patients at increased risk of developing or are diagnosed with TB.
Question 6
In patients with early RA with moderate or high disease activity, who have failed traditional DMARD therapy, should we use TNFi + MTX therapy versus triple DMARD therapy?
Health effects
The panel members judged that the balance between benefits and harms probably favors TNFi + MTX therapy compared to triple DMARD therapy.
Contextual factors
The panel members judged the intervention to be associated with large resource requirements and a reduction in health equities, and to be probably acceptable and probably feasible by key stakeholders.
Recommendation 6
In patients with early RA with moderate or high disease activity, who have failed traditional DMARD therapy, the panel suggests using either TNFi + MTX or triple DMARD therapy (conditional recommendation, very low certainty evidence).
Conditions:
-
Engage patients and consider their preferences when making the choice; TNFi therapy could be prescribed for patients who prefer injections rather than multiple pill intake.
-
Take into consideration the availability of resources.
-
Consider the additional possible harms of TNFi in patients at increased risk of developing or are diagnosed with TB.
Question 7
In patients with early RA with moderate or high disease activity, who have failed traditional DMARD therapy, should we use TNFi monotherapy versus non-TNF biologic therapy?
Health effects
The panel members agreed that the balance between the benefits and harms favors neither the intervention (TNFi monotherapy) nor the comparator (non-TNFi monotherapy).
Contextual factors
The panel members considered that the resource requirements for the intervention vary depending on the weight of the patient, on the mode of administration, and on the cost of the medication. Accordingly, the panel members judged that the impact on health equity would vary. They judged the intervention to be feasible and probably acceptable by key stakeholders.
Recommendation 7
In patients with early RA with moderate or high disease activity, who have failed traditional DMARD therapy, the panel suggests using either TNFi monotherapy or tocilizumab (conditional recommendation, low certainty evidence).
Conditions:
-
Consider that some TNFi therapies cannot be used as mono therapies.
-
Consider the local price/ cost when choosing the therapy
Question 8
In patients with early RA with moderate or high disease activity, who have failed traditional DMARD therapy, should we use TNFi + MTX versus non-TNF biologic (abatacept specifically) + MTX?
Health effects
The panel members judged the balance between the benefits and harms favored neither the intervention (TNFi + MTX therapy) nor the comparator (non-TNFi + MTX).
Contextual factors
The panelists judged that the resource requirements for the intervention vary depending on the weight of the patient, on the mode of administration, and on the cost of the medication. Accordingly, the panel members considered that the impact on health equity will vary. The panel members judged the intervention to be feasible and probably acceptable by key stakeholders.
Recommendation 8
In patients with early RA with moderate or high disease activity, who have failed traditional DMARD therapy, the panel suggests using either TNFi + MTX or abatacept + MTX (conditional recommendation, very low certainty evidence).
Established RA recommendations
Results of the general search for wave 2
Our search for systematic reviews of effectiveness yielded 1304 papers, nine [22, 27, 29,30,31,32,33,34,35] reports were relevant (Appendix g). Similar to wave 1, the five identified studies addressing patients values [36,37,38,39,40] were not relevant the EMR. Studies on resources use identified none relevant to the EMR. However, we retained six studies assessing cost effectiveness of treatment versus comparators [27, 33, 41,42,43,44]. The EtDs can be found in Appendix h.
Wave 2 recommendations (established RA)
Question 1
In patients with established RA, should we use a treat-to-target strategy vs. a non-targeted approach?
Health effects
The panel judged that the balance between the desirable and undesirable effects probably favors the treat-to-target approach.
Contextual factors
The panelists judged the intervention to be probably acceptable to key stakeholders and feasible to implement. However, they judged that the impact of health inequity varies presumably due to cost. They were unclear regarding the resources requirements.
Recommendation 1
In patients with established RA, the panel suggests using a treat to target strategy versus a non-targeted approach (conditional recommendation, moderate certainty evidence).
Question 2
In patients with established RA with moderate or high disease activity, who have failed traditional DMARD therapy, should we use TNFi therapy + MTX vs. combination triple DMARD therapy?
Health effects
The majority of the panelist judged that the desirable consequences probably outweigh undesirable consequences in most settings.
Contextual factors
The panelists judged the intervention to be associated with large resource requirements, probably acceptable by key stakeholders, and probably feasible to implement. However, they judged that the impact of health inequity varies.
Recommendation 2
In patients with established RA with moderate or high disease activity, who have failed traditional DMARD therapy, the panel suggests using TNFi therapy + MTX versus combination triple DMARD therapy (conditional recommendation, very low certainty of evidence).
Conditions:
-
Consider precautions and contraindications of TNFi therapy when switching patients from traditional DMARD therapy.
-
Consider patients with co-morbidities on poly-pharmacy and the patient preferences (e.g., frequency and mode of administration).
-
Suggest using a shared decision-making process between the physician and the patient to better select the appropriate treatment.
Question 3
In patients with established RA with moderate or high disease activity, who have failed multiple TNFi therapies, should we use a non-TNFi biologic therapy + MTX vs. another TNFi + MTX?
Health effects
The panel judged that the balance between the desirable and undesirable effects probably favors the intervention (non-TNFi biologic + MTX).
Contextual factors
The panelists judged the intervention to be associated with negligible costs and savings, probably acceptable by key stakeholders, and probably feasible to implement. However, they judged that the impact of health inequity varies.
Recommendation 3
In patients with established RA with moderate or high disease activity, who have failed multiple TNFi therapies, the panel suggests using non-TNF + MTX biologic therapy over another TNFi + MTX (conditional recommendation, low certainty of evidence).
Condition:
-
Take into consideration whether primary failure of multiple TNFi therapies was due to efficacy failure or to side effects.
Question 4
In patients with established RA with moderate or high disease activity, who have failed a single TNFi therapy, should we use of non-TNF biologic therapy vs. another TNFi?
Health effects
The panel judged, based on low certainty of evidence, that the balance between the desirable and undesirable effects did favor neither the intervention nor the comparator.
Contextual factors
The panelists judged the intervention to be associated with negligible costs and savings, acceptable by key stakeholders, probably feasible to implement, and probably reduced impact on healthy equity.
Recommendation 4
In patients with established RA with moderate or high disease activity, who have failed a single TNFi therapy and are currently on DMARD therapy, the panel suggests using non-TNF biologic therapy over another TNFi (conditional recommendation, low certainty of evidence).
Conditions:
-
Consider using second TNFi if the patient has secondary efficacy failure of first TNFi (consider class switch of TNFi: receptor antagonist vs. monoclonal antibody)
-
Consider using non-TNF biologic if the primary failure of single TNFi therapy was due to efficacy failure or to side effects.
Question 5
In patients with established RA with moderate or high disease activity, who have failed a single TNFi therapy and currently on DMARD, should we use non-TNF biologic therapy + MTX vs. another TNFi + MTX?
Health effects
The panel judged, based on very low certainty of evidence, that the balance between the desirable and undesirable effects did favor neither the intervention nor the comparator.
Contextual factors
The panelists judged the intervention to be associated with: negligible costs and savings, probably acceptable by key stakeholders, probably feasible to implement. However, they judged that the impact of health inequity varies.
Recommendation 5
In patients with established RA with moderate or high disease activity who have failed multiple TNFi therapies, the panel suggests using a non-TNF biologic therapy + MTX versus another TNFi + MTX (conditional recommendation, very low certainty of evidence).
Question 6
In patients with established RA with moderate or high disease activity, who have failed multiple TNFi therapies, should we use oral tofacitinib therapy + MTX vs. another TNFi + MTX?
Health effects
The panel judged, based on low certainty of evidence, that the balance between the desirable and undesirable effects probably favors the intervention (oral tofacitinib + MTX).
Contextual factors
The panelists judged the resource requirements for the intervention to have moderate savings, but there were no studies to assess cost effectiveness. The panel judged the impact on equity to vary. They also judged the intervention to be probably acceptable and feasible.
Recommendation 6
In patients with established RA with moderate or high disease activity who have failed multiple TNFi therapies, the panel suggests using oral tofacitinib therapy + MTX versus another TNFi + MTX (Conditional recommendation, very Low certainty of evidence).
Question 7
In patients with established RA with moderate or high disease activity, should we add versus not add long-term low-dose glucocorticoid therapy to traditional DMARD therapy?
Health effects
The panel judged, based on low certainty of evidence, that the balance between the desirable and undesirable effects probably favors the comparator versus the intervention.
Contextual factors
The panel judged the resource requirements to be negligible in terms of costs or savings for either but the cost effectiveness could not be assessed, as there were no included studies. The panel judged the impact on equity to probably increase and for the intervention to be probably feasible but to have varying acceptability.
Recommendation 7
In patients with established RA, with moderate or high disease activity, the panel suggests against using long-term-low-dose glucocorticoids + traditional DMARD therapy compared to traditional DMARD without glucocorticoids (conditional recommendation, low certainty of evidence).
Question 8
In patients with established RA with moderate or high disease activity with an acute disease flare (RA flare), should we add versus not add short-term high-dose glucocorticoid therapy to traditional DMARDs?
Health effects
The panel judged, based on very low certainty of evidence, that the balance between the desirable and undesirable effects probably favors the intervention versus the comparator.
Contextual factors
The panel judged the resource requirements to be negligible in terms of costs or savings for either but the cost effectiveness of the intervention was judged to probably favor the intervention over the comparator. The panel judged the impact on equity to probably increase and for the intervention to be probably feasible and acceptable.
Recommendation 8
In patients with established RA with moderate or high disease activity, who are experiencing an acute disease flare, the panel suggests using short-term-high-dose glucocorticoids + traditional DMARD therapy compared to traditional DMARD therapy alone (conditional recommendation, very low certainty of evidence).
Discussion
Clinical practice guidelines developed based on the GRADE approach are meant to ensure the provision of optimal patient care taking into account the best available evidence and other factors, such as the availability, feasibility, cost of treatment, the patient values and preferences, and equity issues. The adolopment of guidelines can help achieve this goal, and the project described herein is a proof-of-concept for the value of adoption, adaptation, and de novo development of existing guidelines. Indeed, we were able to successfully adolop in two waves 16 recommendations for the management of RA in the EMR.
The process proved to be feasible, with each wave of adaptation lasting 6 months, and requiring a total budget of ~40,000 USD. The feasibility was facilitated by using existing systematic reviews and collaborating with ACR, the source guideline organization [10]. A key factor in ensuring the success of the project was the high level of expertise on both the content and methodological levels.
Also, the adolopment of the original guidelines led to major changes in the recommendations, as the strength of recommendation changed from strong to conditional for five of the eight early RA adapted recommendation, and two of the eight established RA adapted recommendations (Table 6). The process showed the sensitivity of the strength of recommendation to contextual factors. The change in strength was mainly related to cost of medications, impact on health equities, and acceptability, which are of paramount importance in the EMR. Biologics may not be available or affordable in several of the countries represented by the content experts on the panel.
One limitation of our process relates to how we considered values and preferences in adapting the recommendations. We were able to recruit a patient representative only for the second wave. Also, while we conducted a thorough search for all of the selected questions, the search did not generate valuable information that reflect patient values and preferences within the EMR. As a result, the panel judged that there is probably no uncertainty or variability in how much people value the main outcomes, across all guideline questions. The certainty in this judgment is not as high due to the insufficient data. In fact, patient preferences are unknown in the region and may possibly differ depending on factors such as context, literacy, and health beliefs [45].
In conclusion, this document provides adoloped recommendations for the treatment of early and established RA with moderate to severe activity in the EMR. All the formulated recommendations were conditional recommendations highlighting the need for assessing and evaluating local factors and patient values and preferences.
Change history
06 September 2018
In the original version of this article the first name of the co-author was incorrectly spelled as “Khaled A. Alnaqbi”. The correct spelling should have been “Khalid A. Alnaqbi”. This is now presented correctly in this article.
Abbreviations
- ACR:
-
American college of rheumatology
- AE:
-
Adverse events
- DMARD:
-
Disease-modifying antirheumatic drugs
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- MTX:
-
Methotrexate
- RA:
-
Rheumatoid arthritis
- TNF:
-
Tumor necrosis factor
- TNFi:
-
Tumor necrosis factor alpha inhibitor
References
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324
Chatzidionysiou K, Emamikia S, Nam JL, Ramiro S, Smolen J, Van Der Heijde D, Dougados M, Bijlsma JWJ, Burmester G, Scholte-Voshaar M, Van Vollenhoven R, Landewe R (2016) Efficacy and safety of conventional and targeted synthetic disease-modifying antirheumatic drugs as well as glucocorticoids: a systematic literature review informing the 2016 update of the eular recommendations for the management of rheumatoid arthritis. In: Arthritis and rheumatology. Wiley, Hoboken
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423(6937):356–361
Pincus T (1995) Long-term outcomes in rheumatoid arthritis. Rheumatology 34(suppl 2):59–73
Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, Williams B, Gabriel S, Lassere M, Johns N (2014) The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 73(7):1316–1322
Rasch EK, Hirsch R, Paulose-Ram R, Hochberg MC (2003) Prevalence of rheumatoid arthritis in persons 60 years of age and older in the United States: effect of different methods of case classification. Arthritis Rheum 48(4):917–926
Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P (2007) New therapies for treatment of rheumatoid arthritis. Lancet 370(9602):1861–1874
Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, Emery P, Gaujoux-Viala C, Gossec L, Nam J (2013) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis: Annrheumdis 2013:204573
Smolen JS, van der Heijde D, Machold KP, Aletaha D, Landewé R (2014) Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann Rheum Dis 73(1):3–5
Darzi A, Harfouche M, Arayssi T, Alemadi S, Alnaqbi KA, Badsha H, Al Balushi F, Elzorkany B, Halabi H, Hamoudeh M, Hazer W, Masri B, Omair MA, Uthman I, Ziade N, Singh JA, Christiansen R, Tugwell P, Schunemann HJ, Akl EA (2017) Adaptation of the 2015 American College of Rheumatology treatment guideline for rheumatoid arthritis for the Eastern Mediterranean Region: an exemplar of the GRADE Adolopment. Health Qual Life Outcomes 15(1):183. https://doi.org/10.1186/s12955-017-0754-1
Schunemann HJ, Wiercioch W, Brozek J, Etxeandia-Ikobaltzeta I, Mustafa RA, Manja V, Brignardello-Petersen R, Neumann I, Falavigna M, Alhazzani W, Santesso N, Zhang Y, Meerpohl JJ, Morgan RL, Rochwerg B, Darzi A, Rojas MX, Carrasco-Labra A, Adi Y, AlRayees Z, Riva J, Bollig C, Moore A, Yepes-Nunez JJ, Cuello C, Waziry R, Akl EA (2017) GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol 81:101–110. https://doi.org/10.1016/j.jclinepi.2016.09.009
Alonso-Coello P, Schunemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD (2016) GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: introduction. BMJ (Clinical research ed) 353:i2016. https://doi.org/10.1136/bmj.i2016
Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Vandvik PO, Meerpohl J, Guyatt GH, Schunemann HJ (2016) GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: clinical practice guidelines. BMJ (Clinical research ed) 353:i2089. https://doi.org/10.1136/bmj.i2089
Davoli M, Moja L, Amato L, Ferroni E, Tirani M (2015) Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. Recenti Prog Med 106(6):249–280
Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH (2016) 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheum 68(1):1–26
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schunemann HJ (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64(4):383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026
Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, Johnston BC, Karanicolas P, Akl EA, Vist G, Kunz R, Brozek J, Kupper LL, Martin SL, Meerpohl JJ, Alonso-Coello P, Christensen R, Schunemann HJ (2013) GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol 66(2):173–183. https://doi.org/10.1016/j.jclinepi.2012.08.001
Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, Scholten R, Langendam M, Leeflang MM, Akl EA (2016) GRADE guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol 76:89–98
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64(4):401–406
Guyatt GH, Alonso-Coello P, Schunemann HJ, Djulbegovic B, Nothacker M, Lange S, Murad MH, Akl EA (2016) Guideline panels should seldom make good practice statements: guidance from the GRADE Working Group. J Clin Epidemiol 80:3–7. https://doi.org/10.1016/j.jclinepi.2016.07.006
Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, Nasser M, Meerpohl J, Post PN, Kunz R (2013) GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 66(7):719–725
Stoffer MA, Schoels MM, Smolen JS, Aletaha D, Breedveld FC, Burmester G, Bykerk V, Dougados M, Emery P, Haraoui B (2015) Evidence for treating rheumatoid arthritis to target: results of a systematic literature search update. Ann Rheum Dis: Annrheumdis 2015:207526
Gaujoux-Viala C, Nam J, Ramiro S, Landewé R, Buch MH, Smolen JS, Gossec L (2014) Efficacy of conventional synthetic disease-modifying antirheumatic drugs, glucocorticoids and tofacitinib: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis: Annrheumdis 2013:204588
Menon N, Kothari S, Gogna A, Sharma R (2014) Comparison of intra-articular glucocorticoid injections with DMARDs versus DMARDs alone in rheumatoid arthritis. J Assoc Physicians India 62(8):673–676
De Cock D, Vanderschueren G, Meyfroidt S, Joly J, Westhovens R, Verschueren P (2014) Two-year clinical and radiologic follow-up of early RA patients treated with initial step up monotherapy or initial step down therapy with glucocorticoids, followed by a tight control approach: lessons from a cohort study in daily practice. Clin Rheumatol 33(1):125–130
Verschueren P, De Cock D, Corluy L, Joos R, Langenaken C, Taelman V, Raeman F, Ravelingien I, Vandevyvere K, Lenaerts J (2015) Methotrexate in combination with other DMARDs is not superior to methotrexate alone for remission induction with moderate-to-high-dose glucocorticoid bridging in early rheumatoid arthritis after 16 weeks of treatment: the CareRA trial. Ann Rheum Dis 74(1):27–34
Scott DL, Ibrahim F, Farewell V, O’Keeffe AG, Walker D, Kelly C, Birrell F, Chakravarty K, Maddison P, Heslin M (2015) Tumour necrosis factor inhibitors versus combination intensive therapy with conventional disease modifying anti-rheumatic drugs in established rheumatoid arthritis: TACIT non-inferiority randomised controlled trial. BMJ (Clinical research ed) 350:h1046
Heimans L, Wevers-de Boer K, Visser K, Goekoop R, van Oosterhout M, Harbers J, Bijkerk C, Speyer I, de Buck M, de Sonnaville P (2013) A two-step treatment strategy trial in patients with early arthritis aimed at achieving remission: the IMPROVED study. Ann Rheum Dis: Annrheumdis 2013:203243
Nam JL, Takase-Minegishi K, Ramiro S, Chatzidionysiou K, Smolen JS, van der Heijde D, Bijlsma JW, Burmester GR, Dougados M, Scholte-Voshaar M (2017) Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis: Annrheumdis 2016:210713
Zanwar A, Ahmed S, Misra DP, Agarwal V, Sharma A, Wakhlu A, Negi VS (2017) Recent evidence comparing combination of conventional synthetic disease-modifying antirheumatic drugs with biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Indian J Rheumatol 12(2):104
Dougados M, Kissel K, Conaghan PG, Mola EM, Schett G, Gerli R, Hansen MS, Amital H, Xavier RM, Troum O (2014) Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: the ACT-RAY study. Ann Rheum Dis 73(5):803–809
Gottenberg J-E, Brocq O, Perdriger A, Lassoued S, Berthelot J-M, Wendling D, Euller-Ziegler L, Soubrier M, Richez C, Fautrel B (2016) Non–TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: a randomized clinical trial. Jama 316(11):1172–1180
Manders SH, Kievit W, Adang E, Brus HL, Moens HJB, Hartkamp A, Hendriks L, Brouwer E, Visser H, Vonkeman HE (2015) Cost-effectiveness of abatacept, rituximab, and TNFi treatment after previous failure with TNFi treatment in rheumatoid arthritis: a pragmatic multi-centre randomised trial. Arthritis Res Ther 17(1):134
Pope JE, Haraoui B, Rampakakis E, Psaradellis E, Thorne C, Sampalis JS (2013) Treating to a target in established active rheumatoid arthritis patients receiving a tumor necrosis factor inhibitor: results from a real-world cluster-randomized adalimumab trial. Arthritis Care Res 65(9):1401–1409
Harrold LR, Reed GW, Magner R, Shewade A, John A, Greenberg JD, Kremer JM (2015) Comparative effectiveness and safety of rituximab versus subsequent anti–tumor necrosis factor therapy in patients with rheumatoid arthritis with prior exposure to anti–tumor necrosis factor therapies in the United States Corrona registry. Arthritis Res Ther 17(1):256
Dilla T, Rentero M, Comellas M, Lizan L, Sacristán J (2015) Patients’ preferences for rheumatoid arthritis treatments and their participation in the treatment decision-making process. A systematic review of the literature. Value Health 18(7):A652
Huynh TK, Østergaard A, Egsmose C, Madsen OR (2014) Preferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritis. Patient Prefer Adherence 8:93
Navarro-Millán I, Herrinton LJ, Chen L, Harrold L, Liu L, Curtis JR (2016) Comparative effectiveness of etanercept and adalimumab in patient reported outcomes and injection-related tolerability. PLoS One 11(3):e0149781
Nota I, Drossaert CH, Taal E, van de Laar MA (2015) Patients’ considerations in the decision-making process of initiating disease-modifying antirheumatic drugs. Arthritis Care Res 67(7):956–964
Poulos C, Hauber AB, González JM, Turpcu A (2014) Patients’ willingness to trade off between the duration and frequency of rheumatoid arthritis treatments. Arthritis Care Res 66(7):1008–1015
Harnett J, Wiederkehr D, Gerber R, Gruben D, Koenig A, Bourret J (2016) Real-world evaluation of TNF-inhibitor utilization in rheumatoid arthritis. J Med Econ 19(2):101–112
Meissner B, Trivedi D, You M, Rosenblatt L (2014) Switching of biologic disease modifying anti-rheumatic drugs in patients with rheumatoid arthritis in a real world setting. J Med Econ 17(4):259–265
Wailoo A, Hernández Alava M, Scott IC, Ibrahim F, Scott DL (2014) Cost-effectiveness of treatment strategies using combination disease-modifying anti-rheumatic drugs and glucocorticoids in early rheumatoid arthritis. Rheumatology 53(10):1773–1777
Zhou Z-Y, Griffith J, Du EX, Chin D, Betts KA, Ganguli A (2016) Economic burden of switching to a non-tumor necrosis factor inhibitor versus a tumor necrosis factor inhibitor biologic therapy among patients with rheumatoid arthritis. Adv Ther 33(5):807–823
Montori VM, Brito JP, Murad MH (2013) The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. Jama 310(23):2503–2504. https://doi.org/10.1001/jama.2013.281422
Acknowledgements
We would like to thank Ms. Amy Miller from the American College of Rheumatology (ACR) for her support of the project. We would also like to thank Ms. Diana Jubeily, Dr. Karim Mohamed, Ms. Soha Dargham, Ms. Marianthi Kapiri, Ms. Hadil Ashour, and Ms. Rola El-Rassi for their administrative assistance. Dr. Christensen would like to acknowledge the Oak Foundation that supports the Parker Institute, Bispebjerg and Frederiksberg Hospital (OCAY-13-309). This was part of a collaborative project between the Weill Cornell Medical College in Qatar, the Middle East Rheumatoid Arthritis Consortium (MERAC), and the American University of Beirut (AUB) GRADE (Grading of Recommendations, Assessment, Development and Evaluation) Center.
Funding
The Qatar National Research Fund QNRF’s Conference & Workshop Sponsorship Program (CWSP), CWSP8-W-0919-15023, and Weill Cornell Medical College in Qatar funded the first wave of adaptation. The International League of Associations for Rheumatology (ILAR) funded the second wave of adaptation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The original version of this article was revised: The first name of the co-author was incorrectly spelled as “Khaled A. Alnaqbi”. The correct spelling should have been “Khalid A. Alnaqbi”. This is now presented correctly in this article.
Highlights
• One good practice recommendation for healthcare professionals to provide patients with the needed education regarding progression and treatment options for rheumatoid arthritis.
• Eight graded recommendations for the management of early rheumatoid arthritis.
• Eight graded recommendations for the management of established rheumatoid arthritis.
Electronic supplementary material
ESM 1
(DOCX 2298 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Arayssi, T., Harfouche, M., Darzi, A. et al. Recommendations for the management of rheumatoid arthritis in the Eastern Mediterranean region: an adolopment of the 2015 American College of Rheumatology guidelines. Clin Rheumatol 37, 2947–2959 (2018). https://doi.org/10.1007/s10067-018-4245-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4245-5