Abstract
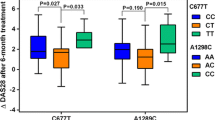
This study investigated the impact of seven polymorphisms in genes of folate transport and (de)glutamation pathway on methotrexate polyglutamate levels and response in patients with rheumatoid arthritis. This prospective study included patients with rheumatoid arthritis. They were treated with methotrexate (up to 25 mg per week) for 24 weeks and categorized by EULAR response criteria into responders (good and moderate) and non-responders. Using real-time Taqman discrimination assay, SNPs were genotyped—rs1045642 (ABCB1 3435C>T), rs1128503 (ABCB1 1236C>T), rs10106 (FPGS 1994A>G), rs1544105 (FPGS G>A), rs11545078 (GGH 452C>T), rs3758149 (GGH -401C>T), and rs1051266 (RFC1 80G>A). RBC methotrexate polyglutamate1–5(MTX-glu1–5) levels were determined at 4, 8, 16, and 24 weeks using by reverse phase HPLC using C-18 column followed by post column photo-oxidation. This study included 117 patients with rheumatoid arthritis (M:F = 14:103). The mean dose of methotrexate at 24 weeks was 22.0 ± 4.0 mg, with data on DAS28(3) at 24 weeks available in 96 patients—61 responders and 35 non-responders. Minor allele of GGH 452C>T had an association with non-response (odds ratio 2.9, 95% CI 1.4–5.6) and assuming the dominance of C, the recessive genetic model found GGH 452C>T CC genotype (odds ratio 9.5, 95% CI 1.2 to 76.0) was significantly associated with response. However, there was no difference in MTX-glu1–5 levels among the various genotypes of this SNP (p = 0.9). Other SNPs were neither associated with response nor with alteration in methotrexate polyglutamate levels. On logistic regression, GGH 452C>T CC genotype and DAS28(3) at baseline were independent predictors of response. GGH 452C>T CC genotype was associated with response to methotrexate. None of the SNPs affected MTX-glu1–5levels.


Similar content being viewed by others
References
Weinblatt ME (2013) Methotrexate in rheumatoid arthritis: a quarter century of development. Trans Am Clin Climatol Assoc 124:16–25
Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, Trentham DE (1985) Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med 312(13):818–822. https://doi.org/10.1056/NEJM198503283121303
Cronstein BN (2005) Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev 57(2):163–172. https://doi.org/10.1124/pr.57.2.3
Tian H, Cronstein BN (2007) Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis 65(3):168–173
Barton A, Worthington J (2009) Genetic susceptibility to rheumatoid arthritis: an emerging picture. Arthritis Rheum 61(10):1441–1446
Romao VC, Canhao H, Fonseca JE (2013) Old drugs, old problems: where do we stand in prediction of rheumatoid arthritis responsiveness to methotrexate and other synthetic DMARDs? BMC Med 11:17. https://doi.org/10.1186/1741-7015-11-17
Galivan J, Johnson T, Rhee M, McGuire JJ, Priest D, Kesevan V (1987) The role of folylpolyglutamate synthetase and gamma-glutamyl hydrolase in altering cellular folyl- and antifolylpolyglutamates. Adv Enzym Regul 26:147–155
Chen ZS, Lee K, Walther S, Raftogianis RB, Kuwano M, Zeng H, Kruh GD (2002) Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res 62(11):3144–3150
Koizumi S, Curt GA, Fine RL, Griffin JD, Chabner BA (1985) Formation of methotrexate polyglutamates in purified myeloid precursor cells from normal human bone marrow. J Clin Invest 75(3):1008–1014. https://doi.org/10.1172/jci111761
Kremer JM (2004) Toward a better understanding of methotrexate. Arthritis Rheum 50(5):1370–1382. https://doi.org/10.1002/art.20278
Mohamed HJ, Sorich MJ, Kowalski SM, McKinnon R, Proudman SM, Cleland L, Wiese MD (2015) The role and utility of measuring red blood cell methotrexate polyglutamate concentrations in inflammatory arthropathies—a systematic review. Eur J Clin Pharmacol 71(4):411–423. https://doi.org/10.1007/s00228-015-1819-x
Kato T, Hamada A, Mori S, Saito H (2012) Genetic polymorphisms in metabolic and cellular transport pathway of methotrexate impact clinical outcome of methotrexate monotherapy in Japanese patients with rheumatoid arthritis. Drug Metab Pharmacokinet 27(2):192–199
Dervieux T, Furst D, Lein DO, Capps R, Smith K, Caldwell J, Kremer J (2005) Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentred cross sectional observational study. Ann Rheum Dis 64(8):1180–1185. https://doi.org/10.1136/ard.2004.033399
Sandhu A, Dhir V, Bhatnagar A, Dhawan V, Kaur J, Sood A, Naidu S, Ahmad S, Varma N, Sharma A, Sharma S (2017) High methotrexate triglutamate level is an independent predictor of adverse effects in Asian Indian rheumatoid arthritis patients—a preliminary study. Ther Drug Monit 39(2):157–163. https://doi.org/10.1097/ftd.0000000000000375
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS et al (1988) The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324
Fransen J, van Riel PL (2005) The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol 23(5 Suppl 39):S93–S99
Sharma S, Das M, Kumar A, Marwaha V, Shankar S, Aneja R, Grover R, Arya V, Dhir V, Gupta R, Kumar U, Juyal RC, KT B (2008) Interaction of genes from influx-metabolism-efflux pathway and their influence on methotrexate efficacy in rheumatoid arthritis patients among Indians. Pharmacogenet Genomics 18(12):1041–1049
Hashiguchi M, Shimizu M, Hakamata J, Tsuru T, Tanaka T, Suzaki M, Miyawaki K, Chiyoda T, Takeuchi O, Hiratsuka J, Irie S, Maruyama J, Mochizuki M (2016) Genetic polymorphisms of enzyme proteins and transporters related to methotrexate response and pharmacokinetics in a Japanese population. J Pharm Health Care Sci 2:35. https://doi.org/10.1186/s40780-016-0069-0
Sole X, Guino E, Valls J, Iniesta R, Moreno V (2006) SNPStats: a web tool for the analysis of association studies. Bioinformatics 22(15):1928–1929. https://doi.org/10.1093/bioinformatics/btl268
van der Straaten RJ, Wessels JA, de Vries-Bouwstra JK, Goekoop-Ruiterman YP, Allaart CF, Bogaartz J, Tiller M, Huizinga TW, Guchelaar HJ (2007) Exploratory analysis of four polymorphisms in human GGH and FPGS genes and their effect in methotrexate-treated rheumatoid arthritis patients. Pharmacogenomics 8(2):141–150
Lee YH, Bae SC, Song GG (2016) Association of the ABCB1 C3435T polymorphism with responsiveness to and toxicity of DMARDs in rheumatoid arthritis: a meta-analysis. Z Rheumatol 75(7):707–715. https://doi.org/10.1007/s00393-015-1618-x
Li X, Hu M, Li W, Gu L, Chen M, Ding H, Vanarsa K, Du Y (2016) The association between reduced folate carrier-1 gene 80G/a polymorphism and methotrexate efficacy or methotrexate related-toxicity in rheumatoid arthritis: a meta-analysis. Int Immunopharmacol 38:8–15. https://doi.org/10.1016/j.intimp.2016.05.012
Ghodke-Puranik Y, Puranik AS, Shintre P, Joshi K, Patwardhan B, Lamba J, Niewold TB, Chopra A (2015) Folate metabolic pathway single nucleotide polymorphisms: a predictive pharmacogenetic marker of methotrexate response in Indian (Asian) patients with rheumatoid arthritis. Pharmacogenomics 16(18):2019–2034. https://doi.org/10.2217/pgs.15.145
Cheng Q, Wu B, Kager L, Panetta JC, Zheng J, Pui CH, Relling MV, Evans WE (2004) A substrate specific functional polymorphism of human gamma-glutamyl hydrolase alters catalytic activity and methotrexate polyglutamate accumulation in acute lymphoblastic leukaemia cells. Pharmacogenetics 14(8):557–567
Cheng Q, Cheng C, Crews KR, Ribeiro RC, Pui CH, Relling MV, Evans WE (2006) Epigenetic regulation of human gamma-glutamyl hydrolase activity in acute lymphoblastic leukemia cells. Am J Hum Genet 79(2):264–274
Acknowledgements
Methotrexate tablets were received as an educational grant from Zydus Activa (Zydus Cadilla Inc., Ahmedabad, India). We acknowledge the efforts of Mrs. Nidhi Gupta and Mr. Mohinder Kumar for sample collection. We also acknowledge the all technical staff of central sophisticated cell of PGIMER for the use of instruments.
Funding
This work was funded by a grant from the Asia Pacific League of Associations for Rheumatology (APLAR) (gene polymorphisms) and from the Department of Biotechnology, Government of India [Grant # BT/PR4608/MED/30/800/2012] (methotrexate polyglutamate levels). Mr. Amit Sandhu was supported by ICMR through its Junior Research Fellowship scheme. Mr. Shabeer Ahmad was supported through a Junior Research Fellowship from the Department of Science and Technology (DST), Government of India.
Author information
Authors and Affiliations
Contributions
Planning study: VDhir, AS; collecting data: AS, VDhir; laboratory work: AS, VDhir, VDhaw, JK, AB; analysis and writing: AS, VDhir, VDhaw, JK, AB.
Corresponding author
Ethics declarations
Disclosures
None.
Ethics approval and consent to participate
This study was approved by the institutional ethics committee of Post Graduate Institute of Medical Education and Research. All patients gave written informed consent.
Rights and permissions
About this article
Cite this article
Sandhu, A., Ahmad, S., Kaur, J. et al. Do SNPs in folate pharmacokinetic pathway alter levels of intracellular methotrexate polyglutamates and affect response? A prospective study in Indian patients. Clin Rheumatol 37, 3221–3228 (2018). https://doi.org/10.1007/s10067-018-4206-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4206-z