Abstract
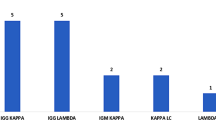
To analyze the clinical spectrum, laboratory characteristics, and outcomes of monoclonal gammopathy (MG) in patients with rheumatic diseases. Screening for the presence of MG was performed in 872 inpatients with rheumatic diseases from January 2010 to July 2017. A total of 41 patients were enrolled. Their clinical and biological features in addition to outcomes were described. For each patient with primary Sjögren syndrome (pSS), 2 age- and sex-matched pSS patients without MG were selected as controls. Risk factors for the presence of MG and malignant hematological neoplasias were assessed. MG was observed in patients with SS, rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, primary biliary cirrhosis, polymyositis, hypomyopathic dermatomyositis, psoriatic arthritis, ANCA-associated vasculitis, polyarteritis nodosa, and polymyalgia rheumatic, with SS the most frequent type. Serum M protein was detected in 37 patients. The monoclonal bands identified in serum were 16 IgG (5 κ, 11 λ), 11 IgA (6 κ, 5 λ), 6 IgM (5 κ, 1 λ), and 4 free λ chains. M components were observed in urine in the other 4 patients. High ESR, albumin/globulin inversion, rheumatoid factor positivity, hypergammaglobulinemia, and hypocomplementemia were common features, presented in more than half of the 41 patients. Patients with pSS, when complicated with MG, showed a higher rate of abnormal urine NAG (71.4 vs 15.8%, P = 0.025), higher levels of ESR [55.0 (53.5) mm/h vs 21.0 (31.8) mm/h, P = 0.001], ESSDAI [26.0 (25.0) vs 12.0 (9.0), P = 0.006], and ClinESSDAI scores [24.0 (25.0) vs 10.5 (10.0), P = 0.011]. Multivariate analysis revealed that the disease activity, assessed by either ESSDAI [adjusted OR 1.127 (95%CI 1.015–1.251), P = 0.025] or ClinESSDAI [adjusted OR 1.121 (95%CI 1.011–1.242), P = 0.030], was the only independent risk factor for the presence of MG. During the follow-up, 2 patients had transient serum M protein, 2 had isotype switch, 1 progressed to multiple myeloma (MM), and another 2 experienced renal injuries attributed by monoclonal or polyclonal plasma cell interstitial infiltration. Seven (17.1%) of the 41 MG patients presented hematological neoplasias, 4 with MM, 2 with smoldering multiple myeloma, and 1 with B cell lymphoma of mucosa-associated lymphoid tissue (MALT) type. The presence of light-chain MG was associated with the development of MM [OR 17.5 (95%CI 1.551–197.435), P = 0.041], but not with an increased risk of lymphoma or SMM. MG was observed in patients with various rheumatic disorders, with SS being the most common type. The presence of MG might be associated with higher disease activity. The development of hematological neoplasias including MM and lymphoma was seen in this setting. Therefore, we recommend the screening for MG and close monitoring for potential malignant transformation in patients with rheumatic diseases as needed.

Similar content being viewed by others
References
International Myeloma Working Group (2003) Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 121(5):749–757. https://doi.org/10.1046/j.1365-2141.2003.04355.x
Raposo A, Peixoto D, Bogas M (2014) Monoclonal gammopathy and rheumatic diseases. Acta Reumatol Port 39(1):12–18
Anderson LA, Gadalla S, Morton LM, Landgren O, Pfeiffer R, Warren JL, Berndt SI, Ricker W, Parsons R, Engels EA (2009) Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int J Cancer 125(2):398–405. https://doi.org/10.1002/ijc.24287
Kassan SS, Thomas TL, Moutsopoulos HM, Hoover R, Kimberly RP, Budman DR, Costa J, Decker JL, Chused TM (1978) Increased risk of lymphoma in sicca syndrome. Ann Intern Med 89(6):888–892. https://doi.org/10.7326/0003-4819-89-6-888
Theander E, Henriksson G, Ljungberg O, Mandl T, Manthorpe R, Jacobsson LT (2006) Lymphoma and other malignancies in primary Sjögren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis 65(6):796–803. https://doi.org/10.1136/ard.2005.041186
Landgren O, Linet MS, McMaster ML, Gridley G, Hemminki K, Goldin LR (2006) Familial characteristics of autoimmune and hematologic disorders in 8,406 multiple myeloma patients: a population-based case-control study. Int J Cancer 118(12):3095–3098. https://doi.org/10.1002/ijc.21745
Fallah M, Liu X, Ji J, Försti A, Sundquist K, Hemminki K (2014) Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol 25(10):2025–2030. https://doi.org/10.1093/annonc/mdu365
Hemminki K, Liu X, Försti A, Ji J, Sundquist J, Sundquist K (2012) Effect of autoimmune diseases on incidence and survival in subsequent multiple myeloma. J Hematol Oncol 5:59. https://doi.org/10.1186/1756-8722-5-59
Weng MY, Huang YT, Liu MF, Lu TH (2012) Incidence of cancer in a nationwide population cohort of 7852 patients with primary Sjögren’s syndrome in Taiwan. Ann Rheum Dis 71(4):524–527. https://doi.org/10.1136/annrheumdis-2011-200402
Zhang W, Feng S, Yan S, Zhao Y, Li M, Sun J, Zhang FC, Cui Q, Dong Y (2010) Incidence of malignancy in primary Sjögren’s syndrome in a Chinese cohort. Rheumatology (Oxford) 49(3):571–577. https://doi.org/10.1093/rheumatology/kep404
Walters MT, Stevenson FK, Herbert A, Cawley MI, Smith JL (1986) Lymphoma in Sjögren’s syndrome: urinary monoclonal free light chains as a diagnostic aid and a means of tumour monitoring. Scand J Rheumatol Suppl 61:114–117
Tomi AL, Belkhir R, Nocturne G, Desmoulins F, Berge E, Pavy S, Miceli-Richard C, Mariette X, Seror R (2016) Brief report: monoclonal gammopathy and risk of lymphoma and multiple myeloma in patients with primary Sjögren’s syndrome. Arthritis Rheumatol 68(5):1245–1250. https://doi.org/10.1002/art.39534
Brito-Zerón P, Ramos-Casals M, Nardi N, Cervera R, Yagüe J, Ingelmo M, Font J (2005) Circulating monoclonal immunoglobulins in Sjögren syndrome: prevalence and clinical significance in 237 patients. Medicine (Baltimore) 84(2):90–97. https://doi.org/10.1097/01.md.0000157398.37679.47
Brito-Zerón P, Retamozo S, Gandía M, Akasbi M, Pérez-De-Lis M, Diaz-Lagares C, Bosch X, Bové A, Pérez-Alvarez R, Soto-Cárdenas MJ, Sisó A, Ramos-Casals M (2012) Monoclonal gammopathy related to Sjögren syndrome: a key marker of disease prognosis and outcomes. J Autoimmun 39(1–2):43–48. https://doi.org/10.1016/j.jaut.2012.01.010
Ali YM, Urowitz MB, Ibanez D, Gladman DD (2007) Monoclonal gammopathy in systemic lupus erythematosus. Lupus 16(6):426–429. https://doi.org/10.1177/0961203307079045
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH, European Study Group on Classification Criteria for Sjögren’s Syndrome (2002) Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61(6):554–558. https://doi.org/10.1136/ard.61.6.554
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA Jr, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG (1998) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324. https://doi.org/10.1002/art.1780310302
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40(9):1725. https://doi.org/10.1002/art.1780400928
Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, Vencovsky J, de Visser M, Hughes RA (2004) 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, the Netherlands. Neuromuscul Disord 14(5):337–345. https://doi.org/10.1016/j.nmd.2004.02.006
Sontheimer RD (2002) Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol 46(4):626–636
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27(4):361–368. https://doi.org/10.1002/art.1780270401
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, CASPAR Study Group (2006) Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 54(8):2665–2673. https://doi.org/10.1002/art.21972
Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ (2009) American Association for Study of Liver Diseases. Primary biliary cirrhosis. Hepatology 50(1):291–308. https://doi.org/10.1002/hep.22906
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA (2013) 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65(1):1–11. https://doi.org/10.1002/art.37715
Lightfoot RW Jr, Michel BA, Bloch DA, Hunder GG, Zvaifler NJ, McShane DJ, Arend WP, Calabrese LH, Leavitt RY, Lie JT, Masi AT, Mills JA, Stevens MB, Wallace SL (1990) The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum 33(8):1088–1093. https://doi.org/10.1002/art.1780330805
Dasgupta B, Cimmino MA, Kremers HM, Schmidt WA, Schirmer M, Salvarani C, Bachta A, Dejaco C, Duftner C, Jensen HS, Duhaut P, Poór G, Kaposi NP, Mandl P, Balint PV, Schmidt Z, Iagnocco A, Nannini C, Cantini F, Macchioni P, Pipitone N, Del Amo M, Espígol-Frigolé G, Cid MC, Martínez-Taboada VM, Nordborg E, Direskeneli H, Aydin SZ, Ahmed K, Hazleman B, Silverman B, Pease C, Wakefield RJ, Luqmani R, Abril A, Michet CJ, Marcus R, Gonter NJ, Maz M, Carter RE, Crowson CS, Matteson EL (2012) 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheum 64(4):943–954. https://doi.org/10.1002/art.34356
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF (2014) International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15(12):e538–e548. https://doi.org/10.1016/S1470-2045(14)70442-5
Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, Gottenberg JE, Bootsma H, Mariette X, Vitali C, EULAR Sjögren’s Task Force (2010) EULAR Sjögren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjögren’s syndrome. Ann Rheum Dis 69(6):1103–1109. https://doi.org/10.1136/ard.2009.110619
Seror R, Meiners P, Baron G, Bootsma H, Bowman SJ, Vitali C, Gottenberg JE, Theander E, Tzioufas A, De Vita S, Ramos-Casals M, Dörner T, Quartuccio L, Ravaud P, Mariette X, EULAR Sjögren Task Force (2016) Development of the ClinESSDAI: a clinical score without biological domain. A tool for biological studies. Ann Rheum Dis 75(11):1945–1950. https://doi.org/10.1136/annrheumdis-2015-208504
Hanawa S, Akimoto T, Uehara E, Inoue M, Imai T, Kotoda A, Yoshizawa H, Matsuyama T, Ueda M, Saito O, Hamano Y, Yumura W, Ozawa K, Muto S, Kusano E (2011) Renal failure caused by plasma cell infiltration in multiple myeloma. Clin Exp Nephrol 15(4):586–590. https://doi.org/10.1007/s10157-011-0437-x
Bridoux F, Leung N, Hutchison CA, Touchard G, Sethi S, Fermand JP, Picken MM, Herrera GA, Kastritis E, Merlini G, Roussel M, Fervenza FC, Dispenzieri A, Kyle RA, Nasr SH, International Kidney and Monoclonal Gammopathy Research Group (2015) Diagnosis of monoclonal gammopathy of renal significance. Kidney Int 87(4):698–711. https://doi.org/10.1038/ki.2014.408
Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, Kröger N, Einsele H, Vesole DH, Dimopoulos M, San Miguel J, Avet-Loiseau H, Hajek R, Chen WM, Anderson KC, Ludwig H, Sonneveld P, Pavlovsky S, Palumbo A, Richardson PG, Barlogie B, Greipp P, Vescio R, Turesson I, Westin J, Boccadoro M, International Myeloma Working Group (2010) Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 24(6):1121–1127. https://doi.org/10.1038/leu.2010.60
Pérez-Persona E, Vidriales MB, Mateo G, García-Sanz R, Mateos MV, de Coca AG, Galende J, Martín-Nuñez G, Alonso JM, de Las Heras N, Hernández JM, Martín A, López-Berges C, Orfao A, San Miguel JF (2007) New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 110(7):2586–2592. https://doi.org/10.1182/blood-2007-05-088443
Kyle RA, Larson DR, Therneau TM, Dispenzieri A, Kumar S, Cerhan JR, Rajkumar SV (2018) Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med 378(3):241–249. https://doi.org/10.1056/NEJMoa1709974
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Research Ethics Committees and carried out in compliance with the Helsinki Declaration. Both of the patient groups gave written informed consent.
Disclosures
None.
Rights and permissions
About this article
Cite this article
Yang, Y., Chen, L., Jia, Y. et al. Monoclonal gammopathy in rheumatic diseases. Clin Rheumatol 37, 1751–1762 (2018). https://doi.org/10.1007/s10067-018-4064-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4064-8