Abstract
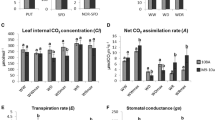
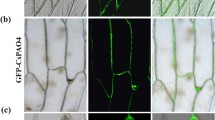
A highly oxidative stress-tolerant japonica rice line was isolated by T-DNA insertion mutation followed by screening in the presence of 50 mM H2O2. The T-DNA insertion was mapped to locus Os09g0547500, the gene product of which was annotated as lysine decarboxylase-like protein (GenBank accession No. AK062595). We termed this gene OsLDC-like 1, for Oryza sativa lysine decarboxylase-like 1. The insertion site was in the second exon and resulted in a 27 amino acid N-terminal deletion. Despite this defect in OsLDC-like 1, the mutant line exhibited enhanced accumulation of the polyamines (PAs) putrescine, spermidine, and spermine under conditions of oxidative stress. The generation of reactive oxygen species (ROS) in the mutant line was assessed by qRT-PCR analysis of NADPH oxidase (RbohD and RbohF), and by DCFH-DA staining. Cellular levels of ROS in osldc-like 1 leaves were significantly lower than those in the wild-type (WT) rice after exposure to oxidative, high salt and acid stresses. Exogenouslyapplied PAs such as spermidine and spermine significantly inhibited the stress-induced accumulation of ROS and cell damage in WT leaves. Additionally, the activities of ROS-detoxifying enzymes were increased in the homozygous mutant line in the presence or absence of H2O2. Thus, mutation of OsLDC-like 1 conferred an oxidative stress-tolerant phenotype. These results suggest that increased cellular PA levels have a physiological role in preventing stress-induced ROS and ethylene accumulation and the resultant cell damage.
Similar content being viewed by others
References
Alcázar, R., Marco, F., Cuevas, J.C., Patron, M., Ferrando, A., Carrasco, P., Tiburcio, A.F., Altabella, T., Chattopadhyay, M.K., Tabor, C.W., et al. (2006). Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 28, 1867–1876.
Allan, A.C., Lapidot, M., Culver, J.N., and Fluhr, R. (2001). An early tobacco mosaic virus-induced oxidative burst in tobacco indicates extracellular perception of the virus coat protein. Plant Physiol. 126, 97–108.
An, S., Park, S., Jeong, D.H., Lee, D.Y., Kang, H.G., Yu, J.H., Hur, J., Kim, S.R., Kim, Y.H., Lee, M., et al. (2003). Generation and analysis of end sequence database for T-DNA tagging lines in rice. Plant Physiol. 133, 2040–2047.
Apel, K., and Hirt, H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399.
Apostol, I., Heinstein, P.F., and Low, P.S. (1989). Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: role in defense and signal transduction. Plant Physiol. 90, 109–116.
Bagni, N., and Tassoni, A. (2001). Biosynthesis, oxidation and conjugation of aliphatic polyamines in higher plants. Amino Acids 20, 301–317.
Bagni, N., Altamura, M.M., Biondi, S., Mengoli, P., and Torrigiani, P. (1993). Polyamines and morphogenesis in normal and transgenic plant cultures. In Morphogenesis in Plants, Molecular Approaches, K.A. Roubelakis-Angelakis and K. Tran Than Van, eds. (Plenum Press, New York, NY), 89–111.
Biastoff, S., Brandt, W., and Dräger, B. (2009). Putrescine N-methyltransferase — the start for alkaloids. Phytochemistry 70, 1708–1718.
Bors, W., Langebartels, C., Michel, C., and Sandermann, H.J. (1989). Polyamines as radical scavengers and protectants against ozone damage. Phytochemistry 28, 1589–1595.
Bradford, M.M. (1976). A rapid and sensitive method for quantitation of microgram quantities of protein using the principle of proteindye protein-dye binding. Anal. Biochem. 72, 248–254.
Capell, T., Bassie, L., and Christou, P. (2004). Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc. Natl. Acad. Sci. USA 101, 9909–9914.
Chaerle, L., Hagenbeek, D., De Bruyne, E., Valcke, R., Van Der Straeten, D. (2004). Thermal and chlorophyll-fluorescence imaging distinguish plant-pathogen interactions at an early stage. Plant Cell Physiol. 45, 887–896.
Chattopadhyay, M.K., Tabor, C.W., and Tabor, H. (2003). Polyamines protect Escherichia coli cells from the toxic effect of oxygen. Proc. Natl. Acad. Sci. USA 100, 2261–226.
Chattopadhyay, M.K., Tabor, C.W., and Tabor, H. (2006). Polyamine deficiency leads to accumulation of reactive oxygen species in a spe2 Δ mutant of Saccharomyces cerevisiae. Yeast 23, 751–761.
Dat, J.F., Pellinen, R., Beeckman, T., Van De Cotte, B., Langebartels, C., Kangasjärvi, J., Inzé, D., and Van Breusegem, F. (2003). Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco. Plant J. 33, 621–632.
Desikan, R., Hancock, J.T., Bright, J., Harrison, J., Weir, I., Hooley, R., and Neill, S.J. (2005). A role for ETR1 in hydrogen peroxide signaling in stomatal guard cells. Plant Physiol. 137, 831–834.
Durmus, N., and Kadioglu, A. (2005). Spermine and putrescine enhance oxidative stress tolerance in maize leaves. Acta Physiol. Plant. 27, 515–522.
Faust, M., and Wang, S.Y. (1993). Polyamines in horticuturally important plants. Hortic. Rev. 14, 333–356.
Flores, H.E., and Galston, A.W. (1984). Osmotic stress-induced polyamine accumulation in cereal leaves. Physiological parameters of the response. Plant Physiol. 75, 102–109.
Flores, H.E., Protacio, C.M., and Signs, M.W. (1989). Primary and secondary metabolism of polyamines in plants. In Primary and Secondary Metabolism of Plant Cell Cultures, K. H. Newman, W. Barz and E. Reinhard, eds. (Plenum Press, New York, NY), 329–393.
Goossens, A., and Rischer, H. (2007). Implementation of functional genomics for gene discovery in alkaloid producing plants. Phytochem. Rev. 6, 35–49.
Groppa, M.D., and Benavides, M.P. (2008). Polyamines and abiotic stress: recent advances. Amino Acids 34, 35–45.
Ha, H.C., Sirisoma, N.S., Kuppusamy, P., Zweier, J.L., Woster, P.M., and Casero, R.A.Jr. (1998). The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA 95, 11140–11145.
Habig, W.H., Pabst, M.J., and Jakoby, W.B. (1974). Glutathione Stransferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 25, 7130–7139.
Häkkinen, S.T., Tilleman, S., Swiatek, A., De Sutter, V., Rischer, H., Vanhoutte, I., Van Onckelen, H., Hilson, P., Inze, D., Oksman-Caldentey, K., et al. (2007). Functional characterization of genes involved in pyridine alkaloid biosynthesis in tobacco. Phytochemistry 68, 2773–2785.
Jeon, W.B., Allard, S.T., Bingman, C.A., Bitto, E., Han, B.W., Wesenberg, G.E., and Phillips, G.N. Jr. (2006) X-ray crystal structures of the conserved hypothetical protein from Arabidopsis thaliana gene loci At5g11950 and At5g37210. Proteins 65, 1051–1054.
Joo, J.H., Wang, S.Y., Chen, J.G., Jones, A.M., and Fedoroff, N.V. (2005). Different signaling and cell death roles of geterotrimeric G protein alpha and beta subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17, 975–970.
Kim, J.S., Choi, S.H., and Lee, J.K. (2006). Lysine decarboxylase expression by Vibrio vulnificus is induced by SoxR in response to superoxide stress. J. Bacteriol. 188, 8586–8592.
Kim, M.S., Kim, H.S., Kim, H.N., Kim, Y.S., Baek, K.H., Park, Y.I., Joung, H., and Jeon, J.H. (2007). Growth and tuberization of transgenic potato plants expressing sense and antisense sequences of Cu/Zn superoxide dismutase from lily chloroplast. J. Plant Biol. 50, 490–495.
Kim, I.S., Kim, Y.S., and Yoon, H.S. (2012). Rice ASR1 protein with reactive oxygen species scavenging and chaperone-like activities enhances acquired tolerance to abiotic stresses in Saccharomyces cerevisiae. Mol. Cells 33, 285–293.
Koh, S., Lee, S.C., Kim, M.K., Koh, J.H., Lee, S., An, G., Choe, S., and Kim, S.R. (2007). T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant Mol. Biol. 65, 453–466.
Kurakawa, T., Ueda, N., Maekawa, M., Kobayashi, K., Kojima, M., Nagato, Y., Sakakibara, H., and Kyozuka, J. (2007). Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445, 652–655.
Kuroha, T., Tokunaga, H., Kojima, M., Ueda, N., Ishida, T., Nagawa, S., Fukuda, H., Sugimoto, K., and Sakakibara, H. (2009). Func tional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell 21, 3152–3169.
Kusano, T., Berberich, T., Tateda, C., and Takahashi, Y. (2008). Polyamines: essential factors for growth and survival. Planta 228, 367–381.
Livia, S.S., Gábor, K., and Zoltán, S. (2002). Effect of salt stress on free amino acid and polyamine content in cereals. Acta Biol. Szeged. 46, 73–75.
McCord, J.M., and Fridovich, I. (1969). Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244, 6049–6055.
Nakano, Y., and Asada, K. (1987). Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbatedepleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 28, 131–140.
Nambeesan, S., AbuQamar, S., Laluk, K., Mattoo, A.K., Mickelbart, M.V., Ferruzzi, M.G., Mengiste, T., and Handa, A.K. (2012). Polyamines attenuate ethylene-mediated defense responses to abrogate resistance to Botrytis cinerea in tomato. Plant Physiol. 158, 1034–1045.
Ohe, M., Scoccianti, V., Bagni, N., Tassoni, A., and Matsuzaki, S. (2009). Putative occurrence of lysine decarboxylase isoforms in soybean (Glycine man) seedlings. Amino Acids 36, 65–70.
Ormrod, D.P., and Beckerson, D.W. (1986). Polyamines as antiozonants for tomato. HortScience 21, 1070–1071.
Park, K.Y., and Lee, S.H. (1994). Effects of ethylene and auxin on polyamine levels in suspension-cultured tobacco cells. Physiol. Plant. 90, 382–390.
Scalet, M., Federico, R., Guido, M.C., and Manes, F. (1995). Peroxidase activity and polyamine changes in response to ozone and simulated acid rain in Aleppo pine needles. Environ. Exp. Bot. 35, 417–425.
Tkachenko, A., Nesterova, L., and Pshenichnov, M. (2001). The role of the natural polyamine putrescine in defense against oxidative stress in Escherichia coli. Arch Microbiol. 176, 155–157.
Wang, Y., Luo, J.P., Wu, H.Q., and Jin, H. (2009). Conversion of protocorm-like bodies of Dendrobium huoshanense to shoots: the role of polyamines in relation to the ratio of total cytokinins and indole-3-acetic acid. J. Plant Physiol. 166, 2013–2022.
Wi, S.J., and Park, K.Y. (2002). Antisense expression of carnation cDNA encoding ACC synthase or ACC oxidase enhances polyamine content and abiotic stress tolerance in transgenic tobacco plants. Mol. Cells 13, 209–220.
Wi, S.J., Kim, W.T., and Park, K.Y. (2006). Overexpression of carnation S-adenosyl-methionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep. 25, 1111–1121.
Wi, S.J., Ji, N.R., and Park, K.Y. (2012). Synergistic biosynthesis of biphasic ethylene and reactive oxygen species in response to hemibiotrophic Phytophthora parasitica in tobacco plants. Plant Physiol. 159, 251–265.
Yamakawa, H., Kamada, H., Satoh, M., and Ohashi, Y. (1998). Spermine is a salicylate-independent endogenous inducer for both tobacco acidic pathogenesis-related proteins and resistance against tobacco mosaic virus infection. Plant Physiol. 118, 1213–1222.
Zhao, F.G., and Qin, P. (2004). Protective effect of exogenous polyamines on root tonoplast function against salt stress in barley seedlings. Plant Growth Regul. 42, 97–103.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Jang, S.J., Wi, S.J., Choi, Y.J. et al. Increased polyamine biosynthesis enhances stress tolerance by preventing the accumulation of reactive oxygen species: T-DNA mutational analysis of Oryza sativa lysine decarboxylase-like protein 1. Mol Cells 34, 251–262 (2012). https://doi.org/10.1007/s10059-012-0067-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-012-0067-5