Abstract
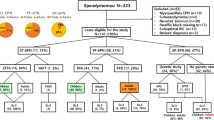
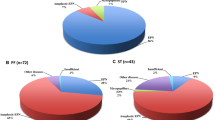
The 2021 WHO classification stratifies ependymoma (EPN) into nine molecular subgroups according to the anatomic locations which outperforms histological grading. We aimed at molecularly reclassifying 200 EPN using immunohistochemistry (IHC) and sequencing for ZFTA fusions in supratentorial (ST) EPN. Further, we assessed the utility of L1CAM, cyclinD1, and p65 markers in identifying ZFTA fusion. Demographic profiles, histologic features, molecular subgroups and clinical outcome were retrospectively analyzed. IHC for L1CAM, cyclinD1, p65, H3K27me3, and H3K27M and sequencing for ZFTA fusion were performed. ZFTA fusions were identified in 44.8% ST EPN. p65 displayed the highest specificity (93.8%), while L1CAM had the highest sensitivity (92.3%) in detecting ZFTA fusions. The negative predictive value approached 96.6% and sensitivity improved to 96.2% with combinatorial IHC (L1CAM, cyclinD1, p65). H3K27me3 loss (PF-A) was noted in 65% PF EPN. Our results provide evidence that a combination of two of three (L1CAM, p65, and cyclinD1) can be used as surrogate markers for predicting fusion. ZFTA fusion, and its surrogate markers in ST, and H3K27me3 and younger age (< 5 years) in PF showed significant correlation with PFS and OS on univariate and Kaplan–Meier analysis. On multivariate analysis, H3K27me3 loss and younger age group are associated with poor clinical outcome.




Similar content being viewed by others
References
Ellison DW, McLendon R, Wiestler OD et al (2016) Ependymal tumors. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella-Branger D, Perry A, Reifenberger G, von Deimling A (eds) WHO classification of tumours of the central nervous system, 4th edn. International Agency for Research on Cancer, Lyon, pp 101–114
McGuire CS, Sainani KL, Fisher PG (2009) Incidence patterns for ependymoma: a surveillance, epidemiology, and end results study. J Neurosurg 110:725–729
Ellison DW, Kocak M, Figarella-Branger D et al (2011) Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed 10:7
Tihan T, Zhou T, Holmes E et al (2008) The prognostic value of histological grading of posterior fossa ependymomas in children: a Children’s Oncology Group study and a review of prognostic factors. Mod Pathol 21:165–177
Gupta K, Salunke P (2017) Understanding ependymoma oncogenesis: an update on recent molecular advances and current perspectives. Mol Neurobiol 54(1):15–21
Pajtler KW, Witt H, Sill M et al (2015) Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell 27(5):728–743
Ellison DW (2022) Ependymoma. In: WHO classification of tumours editorial board. World Health Organization classification of tumours of the central nervous system. 5th edn. International Agency for Research on Cancer press, Lyon, pp 159–176
Parker M, Mohankumar KM, Punchihewa C et al (2014) C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature 506(7489):451–455
Pietsch T, Wohlers I, Goschzik T et al (2014) Supratentorial ependymomas of childhood carry C11orf95-RELA fusions leading to pathological activation of the NF-κB signaling pathway. Acta Neuropathol 127(4):609–611
Ellison DW, Aldape KD, Capper D et al (2020) cIMPACT-NOW update 7: advancing the molecular classification of ependymal tumors. Brain Pathol 30(5):863–866
Andreiuolo F, Varlet P, Tauziède-Espariat A et al (2019) Childhood supratentorial ependymomas with YAP1-MAMLD1 fusion: an entity with characteristic clinical, radiological, cytogenetic and histopathological features. Brain Pathol 29(2):205–216
Torre M, Alexandrescu S, Dubuc AM et al (2020) Characterization of molecular signatures of supratentorial ependymomas. Mod Pathol 33(1):47–56
Malgulwar PB, Nambirajan A, Pathak P et al (2018) C11orf95-RELA fusions and upregulated NF-KB signalling characterise a subset of aggressive supratentorial ependymomas that express L1CAM and nestin. J Neurooncol 138(1):29–39
Gessi M, Giagnacovo M, Modena P et al (2019) Role of immunohistochemistry in the identification of supratentorial C11ORF95-RELA fused ependymoma in routine neuropathology. Am J Surg Pathol 43(1):56–63
Sasaki A, Hirato J, Hirose T et al (2019) Review of ependymomas: assessment of consensus in pathological diagnosis and correlations with genetic profiles and outcome. Brain Tumor Pathol 36(2):92–101
Pagès M, Pajtler KW, Puget S et al (2019) Diagnostics of pediatric supratentorial RELA ependymomas: integration of information from histopathology, genetics, DNA methylation and imaging. Brain Pathol 29(3):325–335
Merchant TE, Bendel AE, Sabin ND et al (2019) Conformal radiation therapy for pediatric ependymoma, chemotherapy for incompletely resected ependymoma, and observation for completely resected, supratentorial ependymoma. J Clin Oncol 37(12):974–983
Fukuoka K, Kanemura Y, Shofuda T et al (2018) Significance of molecular classification of ependymomas: C11orf95-RELA fusion-negative supratentorial ependymomas are a heterogeneous group of tumors. Acta Neuropathol Commun 6(1):134
Witt H, Mack SC, Ryzhova M et al (2011) Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 20(2):143–157
Panwalkar P, Clark J, Ramaswamy V et al (2017) Immunohistochemical analysis of H3K27me3 demonstrates global reduction in group-A childhood posterior fossa ependymoma and is a powerful predictor of outcome. Acta Neuropathol 134(5):705–714
Lim KY, Lee K, Shim Y et al (2022) Molecular subtyping of ependymoma and prognostic impact of Ki-67. Brain Tumor Pathol 39(1):1–13
Ghasemi DR, Sill M, Okonechnikov K et al (2019) MYCN amplification drives an aggressive form of spinal ependymoma. Acta Neuropathol 138(6):1075–1089
Chavali P, Rao S, Palavalasa S et al (2019) L1CAM immunopositivity in anaplastic supratentorial ependymomas: correlation with clinical and histological parameters. Int J Surg Pathol 27(3):251–258
Wani K, Armstrong TS, Jones DT et al (2014) Characterization of L1CAM as a clinical marker for the C11orf95-RELA fusion in supratentorial ependymomas. Neuro Oncol 16(suppl 5):v30–v30
Figarella-Branger D, Lechapt-Zalcman E, Tabouret E et al (2016) Supratentorial clear cell ependymomas with branching capillaries demonstrate characteristic clinicopathological features and pathological activation of nuclear factor-kappaB signaling. Neuro Oncol 18:919–927
Wang L, Liu L, Li H et al (2019) RELA fusion in supratentorial extraventricular ependymomas: a morphologic, immunohistochemical, and molecular study of 43 cases. Am J Surg Pathol 43(12):1674–1681
Zschernack V, Jünger ST, Mynarek M et al (2021) Supratentorial ependymoma in childhood: more than just RELA or YAP. Acta Neuropathol 141(3):455–466
Tauziède-Espariat A, Siegfried A, Nicaise Y et al (2021) Supratentorial non-RELA, ZFTA-fused ependymomas: a comprehensive phenotype genotype correlation highlighting the number of zinc fingers in ZFTA-NCOA1/2 fusions. Acta Neuropathol Commun 9(1):135
Upadhyaya SA, Robinson GW, Onar-Thomas A et al (2019) Molecular grouping and outcomes of young children with newly diagnosed ependymoma treated on the multi-institutional SJYC07 trial. Neuro Oncol 21(10):1319–1330
Lin GL, Monje M (2020) Understanding the deadly silence of posterior fossa A ependymoma. Mol Cell 78(6):999–1001
Mack SC, Witt H, Piro RM et al (2014) Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature 506(7489):445–450
Jain SU, Do TJ, Lund PJ et al (2019) PFA ependymoma-associated protein EZHIP inhibits PRC2 activity through a H3 K27M-like mechanism. Nat Commun 10(1):2146
Michealraj KA, Kumar SA, Kim LJY et al (2020) Metabolic regulation of the epigenome drives lethal infantile ependymoma. Cell 181(6):1329–1345
Gessi M, Capper D, Sahm F et al (2016) Evidence of H3 K27M mutations in posterior fossa ependymomas. Acta Neuropathol 132(4):635–637
Pajtler KW, Wen J, Sill M et al (2018) Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol 136(2):211–226
Acknowledgements
The authors would like to acknowledge and thank Mr. Sandeep Kumar, PhD scholar, for his help in preparation of tissue microarrays.
Funding
This work was partially supported by the Department of Science and Technology, Science and Engineering Research Board (DST-SERB), India (Grant No. EMR/2017/004385).
Author information
Authors and Affiliations
Contributions
DC: material preparation, data collection, analysis including statistics and manuscript writing. KG: study conception, data analysis, critical review of literature, and manuscript writing; TK: interpretation of results and statistical analysis; AS: planning and executing the experiments, data interpretation; PS: data collection, operative details and follow-up; RM and NK: follow-up data collection; BDR: interpretation of results and critical review of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interests.
Ethical approval
This study was in accordance with the ethical standards of the institutional research committee and was approved by the same. The study conforms to the Declaration of Helsinki’s ethical principles. Written informed consent was acquired from all human subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chinnam, D., Gupta, K., Kiran, T. et al. Molecular subgrouping of ependymoma across three anatomic sites and their prognostic implications. Brain Tumor Pathol 39, 151–161 (2022). https://doi.org/10.1007/s10014-022-00429-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-022-00429-2