Abstract
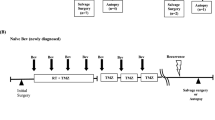
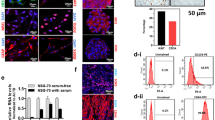
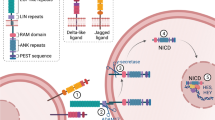
Glioma stem cells (GSCs) have the capacity to repopulate tumors and mediate resistance to radiotherapy and chemotherapy. The Notch signaling pathway is important in proliferation, stem cell maintenance, cell differentiation, and tumorigenesis in GSCs. In this study, we compared CD133, Notch, and VEGF expressions in histological sections of primary and recurrent glioblastomas after radiotherapy and chemotherapy. In vitro study, the γ-secretase inhibitor inhibited NICD, Hes1 and pVEGFR2 expressions in GSCs. GSCs cultured under endothelial conditions undergo endothelial differentiation. Tumor samples were collected from 27 patients at the time of tumor recurrence. We used immunohistochemical techniques to compare expression of CD133, Notch-1 and VEGF. Expressions of CD133-, Notch-1-, and VEGF-positive glioma cells were higher in recurrent glioblastoma after radiotherapy and chemotherapy. To determine the clinical importance of Notch-1 expression in glioblastoma, we analyzed 15 patients who had received bevacizumab therapy followed by a second surgery at recurrence. OS was significantly longer in cases with Notch-1 negativity (8.8 months) than in those with I Notch-1 positivity (6.8 months). We noted that GSCs have the potential for endothelial differentiation with Notch activity. We believe that Notch-1 is a potential target and/or biomarker for antiangiogenic treatments.




Similar content being viewed by others
References
Chang SM, Theodosopoulos P, Lamborn K et al (2004) Temozolomide in the treatment of recurrent malignant glioma. Cancer 100:605–611
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Singh SK, Hawkins C, Clarke ID et al (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Kondo T, Setoguchi T, Taga T (2004) Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA 101:781–786
Ikushima H, Todo T, Ino Y et al (2010) Glioma-initiating cells retain their tumorigenicity through integration of the Sox axis and Oct4 protein. J Biol Chem 286:41434–41441
Bao S, Wu Q, McLendon RE et al (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760
Liu G, Yuan X, Zeng Z et al (2006) Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 5:67
Wurmser AE, Nakashima K, Summers RG et al (2004) Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature 430:350–356
Bao S, Wu Q, Sathornsumetee S et al (2006) Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 66:7843–7848
Folkins C, Shaked Y, Man S et al (2009) Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res 69:7243–7251
Calabrese C, Poppleton H, Kocak M et al (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11:69–82
Gilbertson RJ, Rich JN (2007) Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nature Rev Cancer 7:733–736
Lathia JD, Gallagher J, Heddleston JM et al (2010) Integrin α6 regulates glioblastoma stem cells. Cell Stem Cell 6:421–432
Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137:216–233
Fortini ME (2009) Notch signaling: the core pathway and its posttranslational regulation. Dev Cell 16:633–647
Kageyama R, Ohtsuka T (1999) The Notch-Hes pathway in mammalian neural development. Cell Res 9:179–188
Kanamori M, Kawaguchi T, Nigro JM et al (2007) Contribution of Notch signaling activation to human glioblastoma multiforme. J Neurosurg 106:417–427
Purow BW, Haque RM, Noel MW et al (2005) Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res 65:2353–2363
Zhu TS, Costello MA, Talsma CE et al (2011) Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer Res 71:6061–6072
Wang J, Wakeman TP, Lathia JD et al (2010) Notch promotes radioresistance of glioma stem cells. Stem Cells 28:17–28
Fan X, Khaki L, Zhu TS et al (2010) Notch pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells 28:5–16
Gilbert CA, Daou MC, Moser RP et al (2010) Gamma-secretase inhibitors enhance temozolomide treatment of human gliomas by inhibiting neurosphere repopulation and xenograft recurrence. Cancer Res 70:6870–6879
Saito N, Fu J, Zheng S et al (2014) A high Notch pathway activation predicts response to γ secretase inhibitors in proneural subtype of glioma tumor-initiating cells. Stem Cells 32:301–312
Koul D, Jasser SA, Lu Y et al (2002) Motif analysis of the tumor suppressor gene MMAC/PTEN identifies tyrosines critical for tumor suppression and lipid phosphatase activity. Oncogene 21:2357–2364
Bao S, Wu Q, Li Z et al (2008) Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res 68:6043–6048
Reedijk M, Odorcic S, Zhang H et al (2008) Activation of Notch signaling in human colon adenocarcinoma. Int J Oncol 33:1223–1229
Liu S, Breit S, Danckwardt S et al (2009) Downregulation of Notch signaling by gamma-secretase inhibition can abrogate chemotherapy-induced apoptosis in T-ALL cell lines. Ann Hematol 88:613–621
Watters JW, Cheng C, Majumder PK et al (2009) De novo discovery of a gamma-secretase inhibitor response signature using a novel in vivo breast tumor model. Cancer Res 69:8949–8957
Kageyama R, Ohtsuka T, Hatakeyama J et al (2005) Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res 306:343–348
Hatakeyama J, Sakamoto S, Kageyama R (2006) Hes1 and Hes5 regulate the development of the cranial and spinal nerve systems. Dev Neurosci 28:92–101
Fan X, Mikolaenko I, Elhassan I et al (2004) Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res 64:7787–7793
Nandhu MS, Hu B, Cole SE et al (2014) Novel paracrine modulation of Notch-DLL4 signaling by fibulin-3 promotes angiogenesis in high-grade gliomas. Cancer Res 74:5435–5448
Patenaude A, Fuller M, Chang L et al (2014) Endothelial-specific Notch blockade inhibits vascular function and tumor growth through an eNOS-dependent mechanism. Cancer Res 74:2402–2411
Ricci-Vitiani L, Pallini R, Biffoni M et al (2010) Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468:824–828
Bhat KP, Salazar KL, Balasubramaniyan V et al (2011) The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev 25:2594–2609
Chinot OL, Wick W, Mason W et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370:709–722
Gilbert MR, Dignam JJ, Armstrong TS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699–708
Jordan CT, Guzman ML, Noble M (2006) Cancer stem cells. N Engl J Med 355:1253–1261
Reya T, Morrison SJ, Clarke MF et al (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Baumann M, Krause M, Hill R et al (2008) Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer 8:545–554
Phillips TM, McBride WH, Pajonk F et al (2006) The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst 98:1777–1785
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saito, N., Aoki, K., Hirai, N. et al. Effect of Notch expression in glioma stem cells on therapeutic response to chemo-radiotherapy in recurrent glioblastoma. Brain Tumor Pathol 32, 176–183 (2015). https://doi.org/10.1007/s10014-015-0215-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-015-0215-7