Abstract
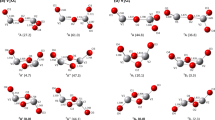
Computations have been performed on VxOy clusters (with x = 1–8, y = 1–21) to explore their structure, stability, and reactivity based on local and global reactivity descriptors defined within the formalism of density functional theory (DFT). The vertical and adiabatic ionization energies and electron affinities are in accordance with Franck–Condon principle and suggest that the VxOy clusters are more likely to be electron acceptors than donors. The structure and reactivity of VxOy clusters delicately depend on their oxygen content and environment. Distinct active sites have been identified for each cluster species on the basis of coordination, symmetry, and charge distribution. The propensity of all the reactive sites towards an approaching electrophile and/or nucleophile has been studied using local reactivity descriptor. In oxygen-poor clusters, the vanadium atoms are more prone to nucleophilic attack. With an increase in oxygen concentration, the coordination number of vanadium increases and reaches four-fold, the site for nucleophilic attack shifts to terminal oxygens. We conclude that of all the stoichiometries, the stable VxOy clusters have the (VO3)a(V2O5)b formula unit. The localization of positive charge density in cubic cage structure of V8O20 successfully traps halide ions (F−, Cl−, and Br−). In view of increasing use of metal oxide clusters in heterogeneous catalysis, the understanding of structure-activity relationship in vanadium oxides’ clusters provided in the current study is highly desirable.











Similar content being viewed by others
References
Muylaert I, Van Der Voort P (2009) Supported vanadium oxide in heterogeneous catalysis: elucidating the structure–activity relationship with spectroscopy. Phys. Chem. Chem. Phys. 11:2826–2832
Chen K, Iglesia E, Bell AT (2000) Kinetic isotopic effects in oxidative dehydrogenation of propane on vanadium oxide catalysts. J. Catal 192:197–203
Lapina OB, Bal'zhinimaev BS, Boghosian S, Eriksen KM, Fehrmann R (1999) Progress on the mechanistic understanding of SO2 oxidation catalysts. Catal. Today 51:469–479
Weckhuysen BM, Keller DE (2003) Chemistry, spectroscopy and the role of supported vanadium oxides in heterogeneous catalysis. Catal. Today 78:25–46
Feyel S, Schröder D, Schwarz H (2006) Gas-phase oxidation of isomeric butenes and small alkanes by vanadium-oxide and-hydroxide cluster cations. J. Phys. Chem. A 110:2647–2654
Feyel S, Döbler J, Schröder D, Sauer J, Schwarz H (2006) Thermal activation of methane by tetranuclear [V4O10]+. Angew. Chem. Int. Ed. 45:4681–4685
Kang H, Beauchamp JL (1986) Gas-phase studies of alkene oxidation by transition-metal oxides. Ion-beam studies of CrO+. J. Am. Chem. Soc. 108:5663–5668
Zemski KA, Justes DR, Bell RC, Castleman AW (2001) Reactions of niobium and tantalum oxide cluster cations and anions with n-butane. J. Phys. Chem. A 105:4410–4417
Dong F, Heinbuch S, Xie Y, Rocca JJ, Bernstein ER, Wang ZC, Deng K, He SG (2008) Experimental and theoretical study of the reactions between neutral vanadium oxide clusters and ethane, ethylene, and acetylene. J. Am. Chem. Soc. 130:1932–1943
Santambrogio G, Brümmer M, Wöste L, Döbler J, Sierka M, Sauer J, Meijer G, Asmis KR (2008) Gas phase vibrational spectroscopy of mass-selected vanadium oxide anions. Phys. Chem. Chem. Phys. 10:3992–4005
Moore NA, Mitrić R, Justes DR, Bonačić-Koutecký V, Castleman AW (2006) Kinetic analysis of the reaction between (V2O5) n= 1, 2+ and ethylene. J. Phys. Chem. B 110:3015–3022
Zhang MQ, ZhaoY X, Liu QY, Li XN, He SG (2016) Does each atom count in the reactivity of vanadia nanoclusters? J. Am. Chem. Soc. 139:42–347
Kooi SE, Castleman Jr AW (1999) Photofragmentation of vanadium oxide cations. J. Phys. Chem. A 103:5671–5674
Bell RC, Zemski KA, Kerns KP, Deng HT, Castleman Jr AW (1998) Reactivities and collision-induced dissociation of vanadium oxide cluster cations. J. Phys. Chem. A 102:1733–1742
Bell RC, Zemski KA, Justes DR, Castleman Jr AW (2001) Formation, structure and bond dissociation thresholds of gas-phase vanadium oxide cluster ions. J. Chem. Phys. 114:798–811
Albaret T, Finocchi F, Noguera C (1999) First principles simulations of titanium oxide clusters and surfaces. Faraday Discuss. 114:285–304
Finocchi F, Noguera C (1996) Structure and bonding of small stoichiometric lithium oxide clusters. Phys. Rev. B 53:4989–4998
Albaret T, Finocchi F, Noguera C (2000) Density functional study of stoichiometric and O-rich titanium oxygen clusters. J. Chem. Phys. 113:2238–2249
Bell RC, Zemski KA, Castleman Jr AW (1999) Gas- phase chemistry of vanadium oxide cluster cations 2. Reactions with CH2F2. J. Phys. Chem. A 103:2992–2998
Bell RC, Zemski KA, Castleman Jr AW (1999) Gas-phase chemistry of vanadium oxide cluster cations 3. Reactions with CCl4. J. Phys. Chem. A 103:1585–1591
Bell RC, Zemski KA, Justes DR, Castleman Jr AW (2001) Formation, structure and bond dissociation thresholds of gas-phase vanadium cluster ions. J. Chem. Phys. 114:798–811
Foltin M, Stueber GJ, Bernstein ER (1999) On the growth dynamics of neutral vanadium oxide and titanium oxide clusters. J. Chem. Phys. 111:9577–9586
Chen ZY, Yang JL (2006) Theoretical study on geometrical and electronic properties of anionic and neutral V2O6 clusters. Chin. J Chem Phys 19:391–394
Miliordos E, Mavridis A (2007) Electronic structure of vanadium oxide. neutral and charged species, VO0,±. J. Phys. Chem. A 111:1953–1965
Jakubikova E, Rappé AK, Bernstein ER (2007) Density functional theory study of small vanadium oxide clusters. J Phys Chem.A 111:12938–12943
Wang LF, Xie L, Fang HL, Li YF, Zhang XB, Wang B, Zhang YF, Huang X (2014) On the structural and electronic properties of hexanuclear vanadium oxide clusters V6On −/0 (n= 12–15): is V6O12 cluster planar or cage-like? Spectrochim. Acta A 131:446–454
Pykavy M, van Wüllen C, Sauer J (2004) Electronic ground states of the V2O4 +/0/− species from multireference correlation and density functional studies. J. Chem. Phys. 120:4207–4215
Matsuda Y, Bernstein ER (2005) Identification, structure, and spectroscopy of neutral vanadium oxide clusters. J. Phys. Chem. A 109:3803–3811
Vyboishchikov SF, Saue J (2000) Gas-phase vanadium oxide anions: structure and detachment energies from density functional calculations. J. Phys. Chem. A 104:10913–10922
Wu JW, Moriyama R, Tahara H, Ohshimo K, Misaizu F (2016) Compositions and structures of vanadium oxide cluster ions VmOn ± (m= 2–20) investigated by ion mobility mass spectrometry. J. Phys. Chem. A 120:3788–3796
Vyboishchikov SF, Sauer J (2001) (V2O5)n Gas-phase clusters (n= 1–12) compared to V2O5 crystal: DFT calculations. J. Phys. Chem. A 105:8588–8598
Jia MY, Xu BK, Deng SG, He MFG (2014) Consecutive oxygen-for-sulfur exchange reactions between vanadium oxide cluster anions and hydrogen sulfide. J. Phys. Chem. A 118:8106–8114
Jakubikova E, Bernstein ER (2007) Reactions of sulfur dioxide with neutral vanadium oxide clusters in the gas phase. I. Density Functional Theory Study. J. Phys. Chem. A 111:13339–13346
Yuan Z, Li ZY, Zhou ZX, Liu QY, Zhao YX, He SG (2014) Thermal reactions of (V2O5)nO− (n= 1–3) cluster anions with ethylene and propylene: oxygen atom transfer versus molecular association. J. Phys. Chem. C 118:14967–14976
Dong F, Heinbuch S, Xie Y, Bernstein ER, Rocca JJ, Wang ZC, Ding XL, He SG (2009) C═C bond cleavage on neutral VO3(V2O5)n clusters. J. Am. Chem. Soc. 131:1057–1066
Wu XN, Ding XL, Li ZY, Zhao YX, He SG (2014) Hydrogen atom abstraction from CH4 by nanosized vanadium oxide cluster cations. J. Phys. Chem. C 118:24062–24071
Parr RG, Szentpaly LV, Liu S (1991) Electrophilicity index. J. Am. Chem. Soc. 121:1922–1924
Parr RG, Donnelly RA, Levy M, Palke WE (1978) Electronegativity: the density functional viewpoint. J. Chem. Phys. 68:3801–3807
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, Oxford
Pearson RG (1963) Hard and soft acids and bases. J. Am. Chem. Soc. 85:3533–3539
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105:7512–7516
Chattaraj PK, Lee H, Parr RG (1991) HSAB principle. J. Am. Chem. Soc. 113:1855–1856
Chattaraj PK, Schleyer PVR (1994) An ab initio study resulting in a greater understanding of the HSAB principle. J. Am. Chem. Soc. 116:1067–1071
Chattaraj PK, Maiti B (2003) HSAB principle applied to the time evolution of chemical reactions. J. Am. Chem. Soc. 125:2705–2710
Pearson RG (1997) Chemical hardness. Wiley-VCH
Berkowitz M, Ghosh SK, Parr RG (1985) On the concept of local hardness in chemistry. J. Am. Chem. Soc. 107:6811–6814
Kohn W, Sham L (1965) Self-consistent equations including exchange and correlation effects. Phys. Rev. 140:1133–1138
Domingo LR, Chamorro E, Pérez P (2008) Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 73:4615–4624
Pérez P, Domingo LR, Duque-Noreña M, Chamorro EA (2009) Condensed-to-atom nucleophilicity index. An application to the director effects on the electrophilic aromatic substitutions. J. Mol. Struct.: Theochem. 895:86–91
Sharma P, Kumar A, Sahu V (2009) Theoretical evaluation of global and local electrophilicity patterns to characterize hetero-Diels− Alder cycloaddition of three-membered 2H-azirine ring system. J. Phys. Chem. A 114:1032–1038
Sharma P, Kumar A, Sahu V (2011) Methyl 2-(4-methylphenyl)-2H-azirine-3-carboxylate as dienophile in hetero-Diels-Alder cycloaddition: a DFT approach. Lett. Org. Chem. 8:132–137
Chattaraj PK, Giri S, Duley S (2011) Update 2 of electrophilicity index. Chem. Rev. 111:43–75
Soto-Delgado J, Domingo LR, Contreras R (2010) Quantitative characterization of group electrophilicity and nucleophilicity for intramolecular Diels–Alder reactions. Org. Biomol. Chem. 8:3678–3683
Domingo LR, Zaragozá RJ, Saéz JA, Arnó M (2012) Understanding the mechanism of the intramolecular Stetter reaction. A DFT study. Molecules 17:1335–1353
Roos G, Geerlings P, Messens J (2009) Enzymatic catalysis: the emerging role of conceptual density functional theory. J. Phys. Chem. B 113:13465–13475
Kar R, Pal S (2010) Effect of solvents having different dielectric constants on reactivity: a conceptual DFT approach. Int. J. Quantum Chem. 110:1642–1647
De HS, Krishnamurty S, Pal S (2009) Density functional investigation of relativistic effects on the structure and reactivity of tetrahedral gold clusters. J. Phys. Chem. C 113:7101–7106
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. Chem. Phys. 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37:785–789
Schäfer A, Huber C, Ahlrichs R (1994) Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 100:5829–5835
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H (2009) Gaussian 09, revision A.1. Gaussian. Inc, Wallingford
Kaur N, Kumari I, Gupta S, Goel N (2016) Spin inversion phenomenon and two-state reactivity mechanism for direct benzene hydroxylation by V4O10 cluster. J. Phys. Chem. A 120:9588–9597
Li H, Li C, Fan H, Yang J (2010) Studies on electronic structures, energetics, and electron affinities of transition metal–benzene complexes and their anions with density functional theory. J. Mol. Struc.-Theochem. 952:67–73
Chandrakumar KRS, Pal S (2002) The concept of density functional theory based descriptors and its relation with the reactivity of molecular systems: a semi-quantitative study. Int. J. Mol. Sci. 3:324–337
Parr RG, Yang W (1984) Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 106:4049–4050
Yang Y, Parr R (1985) Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. G. Proc. Natl. Acad. Sci. U. S. A. 821:6723–6726
Pearson RG (1987) Recent advances in the concept of hard and soft acids and bases. J. Chem. Educ. 64:561–567
Roy RK, Krishnamurty S, Geerlings P, Pal S (1998) Local softness and hardness based reactivity descriptors for predicting intra-and intermolecular reactivity sequences: carbonyl compounds. J. Phys. Chem. A 102:3746–3755
Roy RK, de Proft FD, Geerlings P (1998) Site of protonation in aniline and substituted anilines in the gas phase: a study via the local hard and soft acids and bases concept. J. Phys. Chem. A 102:7035–7040
Morell C, Grand A, Toro-Labbe A (2005) New dual descriptor for chemical reactivity. J. Phys. Chem. A 109:205–212
Bauzá A, Mooibroek TJ, Frontera A (2013) Tetrel-bonding interaction: rediscovered supramolecular force? Angew. Chem. Int. Ed. 52:12317–12321
Wang ZC, Yin S, Bernstein ER (2013) Catalytic oxidation of CO by N2O conducted via the neutral oxide cluster couple VO2/VO3. Phys. Chem. Chem. Phys. 15:10429–10434
Zhao YX, Ding XL, Ma YP, Wang ZC, He SG (2010) Transition metal oxide clusters with character of oxygen-centered radical: a DFT study. Theor. Chem. Acc. 127:449–465
Kaur N, Gupta S, Goel N (2017) Enantioselective synthesis of sulfoxide using an SBA-15 supported vanadia catalyst: a computational elucidation using a QM/MM approach. Phys. Chem. Chem. Phys. 19:25059–25070
Dong F, Heinbuch S, Xie Y, Rocca JJ, Bernstein ER (2009) Reactions of neutral vanadium oxide clusters with methanol. J. Phys. Chem. A 113:3029–3040
Acknowledgements
It is a pleasure to thank Prof. M. Springborg for being a wonderful host during my (N.G) stay at Saarbrucken; discussions with him and Dr. M. Molayem were very helpful in interpreting the results.
Funding
This work was financially supported by Science and Engineering Research Board (SERB), DST, India via grant no. SERB/F/8589/2014–2015.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 908 kb)
Rights and permissions
About this article
Cite this article
Kaur, N., Gupta, S. & Goel, N. Understanding structure-activity relation in VxOy clusters of varied stoichiometry and sizes through conceptual density functional approach. J Mol Model 25, 319 (2019). https://doi.org/10.1007/s00894-019-4168-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4168-3