Abstract
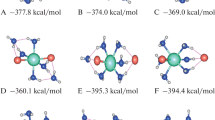
The structures and thermodynamic properties of microhydrates of caesium metaborate (CsBO2) of nuclear safety interest are reported in this work. CsBO2 + n H2O (n = 1−4) molecular complexes were identified on the potential energy surface. The structures were optimized using the ωB97XD DFT method and the aug-cc-pVTZ basis set. Single-point energies were calculated at the CCSD(T)-F12a/awCVTZ and the ωB97XD/aug-cc-pVQZ levels of theory. The standard reaction enthalpies and the standard Gibbs free reaction energies were reported for all molecular complexes. The temperature dependence of ΔrG°(T) was evaluated for all studied structures over the temperature range 300–2000 K. Total hydration reactions were investigated. The results showed that the mono-hydrated form of CsBO2 exists only at temperatures lower than 720 K under standard conditions. The influence on the thermodynamic properties of the number of water molecules in the clusters was described, with successive dehydration from 720 to 480 K. In nuclear severe accident conditions, gaseous CsBO2 will remain unhydrated in the reactor coolant system.






Similar content being viewed by others
References
Li R-Z, Liu C-W, Gao YQ, Jiang H, Xu H-G, Zheng W-J (2013) Microsolvation of LiI and CsI in water: anion photoelectron spectroscopy and ab initio calculations. J Am Chem Soc 135(13):5190–5199. https://doi.org/10.1021/ja4006942
Inada Y, Akagane K (1996) Non-empirical study of chemical reactions including fission products in severe light water reactor accidents. J Nucl Sci Technol 33(7):562–568. https://doi.org/10.1080/18811248.1996.9731956
Inada Y (1998) Non-empirical analysis of the chemical reaction of cesium with steam; Cs+H2O→CsOH+H, in severe light water reactor accidents. J Nucl Sci Technol 35(4):313–316. https://doi.org/10.1080/18811248.1998.9733862
Lee EPF, Wright TG (2003) Theoretical study of RbOH, CsOH, FrOH, and their cations: geometries, vibrational frequencies, and the ionization energies. J Phys Chem A 107(26):5233–5240. https://doi.org/10.1021/jp034747y
Odde S, Pak C, Lee HM, Kim KS, Mhin BJ (2004) Aqua dissociation nature of cesium hydroxide. J Chem Phys 121(1):204–208. https://doi.org/10.1063/1.1757438
Kurosaki Y, Matsuoka L, Yokoyama K, Yokoyama A (2008) Ab initio study on the ground and low-lying excited states of cesium iodide (CsI). J Chem Phys 128(2):024301. https://doi.org/10.1063/1.2821103
Badawi M, Xerri B, Canneaux S, Cantrel L, Louis F (2012) Molecular structures and thermodynamic properties of 12 gaseous cesium-containing species of nuclear safety interest: Cs2, CsH, CsO, Cs2O, CsX, and Cs2X2 (X = OH, Cl, Br, and I). J Nucl Mater 420(1–3):452–462. https://doi.org/10.1016/j.jnucmat.2011.10.034
Sudolská M, Cantrel L, Černušák I (2014) Microhydration of caesium compounds: Cs, CsOH, CsI and Cs2I2 complexes with one to three H2O molecules of nuclear safety interest. J Mol Model 20(4):2218. https://doi.org/10.1007/s00894-014-2218-4
Vandeputte R, Khiri D, Lafont C, Cantrel L, Louis F (2019) Theoretical investigation of thermochemical properties of cesium borates species. J Nucl Mater 517:63–70. https://doi.org/10.1016/j.jnucmat.2019.01.036
Miyahara N, Miwa S, Horiguchi N, Sato I, Masahiko O (2019) Chemical reaction kinetics dataset of Cs-I-B-Mo-O-H system for evaluation of fission product chemistry under LWR severe accident conditions. J Nucl Sci Technol 56(2):228–240. https://doi.org/10.1080/00223131.2018.1544939
Kuczkowski RL, Lide Jr DR, Krisher LC (1966) Microwave spectra of alkali hydroxides : evidence for linearity of CsOH and KOH. J Chem Phys 44(8):3131–3132. https://doi.org/10.1063/1.1727194
Acquista N, Abramowitz S, Lide DR (1968) Structure of the alkali hydroxides. II. The infrared spectra of matrix-isolated CsOH and CsOD. J Chem Phys 49(2):780–782. https://doi.org/10.1063/1.1670139
Story Jr TL, Hebert AJ (1976) Dipole moments of KI, RbBr, RbI, CsBr, and CsI by the electric deflection method. J Chem Phys 64(2):855–858. https://doi.org/10.1063/1.432235
Su TMR, Riley SJ (1979) Alkali halide photofragment spectra. I. Alkali iodide bond energies and excited state symmetries at 266 nm. J Chem Phys 71(8):3194–3202. https://doi.org/10.1063/1.438766
Blackburn PE, Johnson CE (1988) Mass spectrometry studies of fission product behavior: II. Gas phase species in the CsI-CsOH system. J Nucl Mater 154(1):74–82. https://doi.org/10.1016/0022-3115(88)90121-3
Büchler A, Marram EP (1963) Gaseous Metaborates. II. Infrared spectra of alkali metaborate vapors. J Chem Phys 39(2):292–295. https://doi.org/10.1063/1.1734244
Minato K (1991) Thermodynamic analysis of cesium and iodine behavior in severe light water reactor accidents. J Nucl Mater 185(2):154–158. https://doi.org/10.1016/0022-3115(91)90330-A
Miwa S, Yamashita S, Osaka M (2016) Prediction of the effects of boron release kinetics on the vapor species of cesium and iodine fission products. Prog Nucl Energy 92:254–259. https://doi.org/10.1016/j.pnucene.2016.02.023
Lemire RJ, Paquette J, Torgerson DF, Wren DJ, Fletcher JW (1981) Assessment of iodine behaviour in reactor containment buildings from a chemical perspective. Whiteshell Nuclear Research Establishment, Atomic Energy of Canada, Pinawa, Canada
Garisto F (1982) Thermodynamics of iodine, cesium and tellurium in the primary heat-transport system under accident conditions. Whiteshell Nuclear Research Establishment, Atomic Energy of Canada, Pinawa, Canada
Bowsher BR (1987) Fission-product chemistry and aerosol behaviour in the primary circuit of a pressurized water reactor under severe accident conditions. Prog Nucl Energy 20(3):199–233. https://doi.org/10.1016/0149-1970(87)90006-0
Mellor JW (1980) A comprehensive treatise on inorganic and theoretical chemistry: vol. 5, supplement. Boron. Boron–oxygen compounds, part 1. Longmans, London
Elrick RM, Sallach RA, Ouellette AL, Douglas SC (1984) Reaction between some cesium-iodine compounds and the reactor materials 304 stainless steel, inconel 600 and silver. Volume 1: cesium hydroxide reactions. Sandia National Laboratories, Albuquerque, NM
Clément B, Cantrel L, Ducros G, Funke F, Herranz L, Rydl A, Weber G, Wren C (2007) State of the art report on iodine chemistry. Nuclear Energy Agency of the OECD, Paris
Kress TS, Beahm EC, Weber CF, Parker GW (1993) Fission product transport behavior. Nucl Technol 101(3):262–269. https://doi.org/10.13182/nt93-a34789
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16 Rev. A.03. Wallingford, CT
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10(44):6615–6620. https://doi.org/10.1039/b810189b
Dunning Jr TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90(2):1007–1023. https://doi.org/10.1063/1.456153
Hill JG, Peterson KA (2017) Gaussian basis sets for use in correlated molecular calculations. XI. Pseudopotential-based and all-electron relativistic basis sets for alkali metal (K–Fr) and alkaline earth (Ca–Ra) elements. J Chem Phys 147(24):244106. https://doi.org/10.1063/1.5010587
Lim IS, Schwerdtfeger P, Metz B, Stoll H (2005) All-electron and relativistic pseudopotential studies for the group 1 element polarizabilities from K to element 119. J Chem Phys 122(10):104103. https://doi.org/10.1063/1.1856451
NIST computational chemistry comparison and benchmark database (2018) http://cccbdb.nist.gov/
Werner H-J, Knowles PJ, Knizia G, Manby FR, Schütz M, Celani P, Györffy W, Kats D, Korona T, Lindh R, Mitrushenkov A, Rauhut G, Shamasundar KR, Adler TB, Amos RD, Bennie SJ, Bernhardsson A, Berning A, Cooper DL, Deegan MJO, Dobbyn AJ, Eckert F, Goll E, Hampel C, Hesselmann A, Hetzer G, Hrenar T, Jansen G, Köppl C, Lee SJR, Liu Y, Lloyd AW, Ma Q, Mata RA, May AJ, McNicholas SJ, Meyer W, TF Miller III, Mura ME, Nicklaß A, O'Neill DP, Palmieri P, Peng D, Pflüger K, Pitzer R, Reiher M, Shiozaki T, Stoll H, Stone AJ, Tarroni R, Thorsteinsson T, Wang M, Welborn M (2015) Molpro, version 2015, a package of Ab Initio programs. https://www.molpro.net/
Adler TB, Knizia G, Werner H-J (2007) A simple and efficient CCSD(T)-F12 approximation. J Chem Phys 127(22):221106. https://doi.org/10.1063/1.2817618
Knizia G, Adler TB, Werner H-J (2009) Simplified CCSD(T)-F12 methods: theory and benchmarks. J Chem Phys 130(5):054104. https://doi.org/10.1063/1.3054300
Peterson KA, Dunning Jr TH (2002) Accurate correlation consistent basis sets for molecular core–valence correlation effects: the second row atoms Al–Ar, and the first row atoms B–Ne revisited. J Chem Phys 117(23):10548–10560. https://doi.org/10.1063/1.1520138
DeYonker NJ, Peterson KA, Wilson AK (2007) Systematically convergent correlation consistent basis sets for molecular core−valence correlation effects: the third-row atoms gallium through krypton. J Phys Chem A 111(44):11383–11393. https://doi.org/10.1021/jp0747757
Irikura KK (2002) THERMO.PL. National Institute of Standards and Technology, Gaithersburg, MD
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19(4):553–566. https://doi.org/10.1080/00268977000101561
Alvarez-Idaboy JR, Galano A (2010) Counterpoise corrected interaction energies are not systematically better than uncorrected ones: comparison with CCSD(T) CBS extrapolated values. Theor Chem Accounts 126(1):75–85. https://doi.org/10.1007/s00214-009-0676-z
Lebègue P (2019) Gauss2XL version 1.0, a Python software to extract data from Gaussian16 program to excel. Université de Lille
Hoy AR, Bunker PR (1979) A precise solution of the rotation bending Schrödinger equation for a triatomic molecule with application to the water molecule. J Mol Spectrosc 74(1):1–8. https://doi.org/10.1016/0022-2852(79)90019-5
Shimanouchi T (1972) Tables of molecular vibrational frequencies consolidated volume I. National Bureau of Standards
Huber KP, Herzberg G (1979) Molecular spectra and molecular structure. IV. Constants of diatomic molecules. Van Nostrand, New York
Acknowledgments
Computer time for the theoretical calculations was provided by the Centre de Ressources Informatiques (CRI) of the University of Lille and the Centre Régional Informatique et d’Applications Numériques de Normandie (CRIANN). We appreciated the support from the LABEX CaPPA (Chemical and Physical Properties of the Atmosphere), which is funded by the French National Research Agency (ANR) through the PIA (Programme d’Investissement d’Avenir) under contract “ANR-11-LABX-0005-01 and also the Regional Council “Nord-Pas de Calais” and the “European Funds for Regional Economic Development”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 74 kb)
Rights and permissions
About this article
Cite this article
Khiri, D., Vandeputte, R., Taamalli, S. et al. Microhydration of caesium metaborate: structural and thermochemical properties of CsBO2 + n H2O (n = 1–4) aggregates. J Mol Model 25, 207 (2019). https://doi.org/10.1007/s00894-019-4094-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4094-4