Abstract
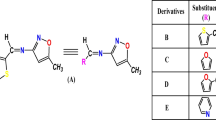
The upper efficiency of heterojunction organic photovoltaics depends on the increased open-circuit voltage (V oc) and short-circuit current (J sc). So, a higher lowest unoccupied molecular orbital (LUMO) level is necessary for organic acceptor material to possess higher V oc and more photons absorbsorption in the solar spectrum is needed for larger J sc. In this article, we theoretically designed some small molecule acceptors (2∼5) based on fluorene (F), benzothiadiazole, and cyano group (CN) referring to the reported acceptor material 2-[{7-(9,9-di-n-propyl-9H-fluoren-2-yl)benzo[c][1,2,5]thiadiazol-4-yl}methylene]malononitrile (1), the crucial parameters affecting photoelectrical properties of compounds 2∼5 were evaluated by the density functional theory (DFT) and time dependent density functional theory (TDDFT) methods. The results reveal that compared with 1, 3 and 4 could have the better complementary absorption spectra with P3HT, the increased LUMO level, the improved V oc, and the decreased electronic organization energy (λ e). From the simulation of transition density matrix, it is very clear that the excitons of molecules 3 and 4 are easier to separate in the material surface. Therefore, 3 and 4 may become potential acceptor candidates for organic photovoltaic cells. In addition, with the increased number of CN, the optoelectronic properties of the molecules show a regular change, mainly improve the LUMO level, energy gap, V oc, and absorption intensity. In summary, reasonably adjusting CN can effectively improve the photovoltaic properties of small molecule acceptors.

Structure–property relationship of small molecule acceptors could be rationally evaluated in the article. The changes of conjugate length and CN are important strategies to alter the photovoltaic properties of small molecule acceptors. Therefore, taking the K12/1 as a reference, we have theoretically designed a series of small molecule acceptors (2–4). The calculated results by means of DFT and TDDFT manifest that molecules 3 and 4 have the better complementary absorption spectra with P3HT, the increased LUMO level, the improved V oc, the decreased electronic organization energy and the easier separation in the material surface than 1. In summary, reasonably increasing conjugate length and decreasing CN can effectively improve the PCE, which will provide a theoretical guideline for the design and synthesis of new small molecule acceptors.





Similar content being viewed by others
References
Brabec CJ, Sariciftci NS, Hummelen JC (2001) Adv Funct Mater 11:15
Cheng YJ, Yang SH, Hsu CS (2009) Chem Rev 109:5868
Friend RH, Gymer RW, Holmes AB, Burroughes JH, Marks RN, Taliani C, Bradley DDC, Dos Santos DA, Brédas JL, Lögdlund M, Salaneck WR (1999) Nature 397:121
Günes S, Neugebauer H, Sariciftci NS (2007) Chem Rev 107:1324
Kularatne RS, Magurudeniya HD, Sista P, Biewer MC, Stefan MC (2013) J Polym Sci Pol Chem 51:743
Darling SB, You F (2013) RSC Adv 3:17633
Kan B, Li M, Zhang Q, Liu F, Wan X, Wang Y, Ni W, Long G, Yang X, Feng H, Zuo Y, Zhang M, Huang F, Cao Y, Russell TP, Chen Y (2015) J Am Chem Soc 137:3886
Kan B, Zhang Q, Li M, Wan X, Ni W, Long G, Wang Y, Yang X, Feng H, Chen Y (2014) J Am Chem Soc 136:15529
Hummelen JC, Knight BW, LePeq F, Wudl F, Yao J, Wilkins CL (1995) J Org Chem 60:532
Wienk MM, Kroon JM, Verhees WJH, Knol J, Hummelen JC, van Hal PA, Janssen RA (2003) J Angew Chem Int Ed 42:3371
Yu G, Gao J, Hummelen JC, Wudl F, Heeger A (1995) J Mar Sci 270:1789
Shin RYC, Kietzke T, Sudhakar S, Dodabalapur A, Chen ZK, Sellinger A (2007) Chem Mater 19:1892
Sonar P, Ng GM, Lin TT, Dodabalapur A, Chen ZK (2010) J Mater Chem 20:3626
Woo CH, Holcombe TW, Unruh DA, Sellinger A, Fréchet JM (2010) J Chem Mater 22:1673
Shu Y, Lim YF, Li Z, Purushothaman B, Hallani R, Kim JE, Parkin SR, Malliaras GG, Anthony JE (2011) Chem Sci 2:363
Mikroyannidis JA, Suresh P, Sharma GD (2010) Org Electron 11:311
Kyaw AKK, Wang DH, Gupta V, Zhang J, Chand S, Bazan GC, Heeger A (2013) J Adv Mater 25:2397
Lin Y, Li Y, Zhan X (2012) Chem Soc Rev 41:4245
Liu Y, Wan X, Wang F, Zhou J, Long G, Tian J, You J, Yang Y, Chen Y (2011) Adv Energy Mater 1:771–775
Sun Y, Welch GC, Leong WL, Takacs CJ, Bazan GC, Heeger A (2012) J Nat Mater 11:44
Zhu XH, Peng J, Cao Y, Roncali J (2011) Chem Soc Rev 40:3509
Schwenn PE, Gui K, Nardes AM, Krueger KB, Lee KH, Mutkins K, Rubinstein-Dunlop H, Shaw PE, Kopidakis N, Burn PL, Meredith P (2011) Adv Energy Mater 1:73–81
Krueger KB, Schwenn PE, Gui K, Pivrikas A, Meredith P, Burn PL (2011) Appl Phys Lett 98:083301
Brunetti FG, Gong X, Tong M, Heeger AJ, Wudl F (2010) Angew Chem Int Ed 49:532
Fang Y, Pandey AK, Nardes AM, Kopidakis N, Burn PL, Meredith P (2013) Adv Energy Mater 3:54
Kim JH, An BK, Yoon SJ, Park SK, Kwon JE, Lim CK, Park SY (2014) Adv Funct Mater 24:2746
Wang H, Li F, Ravia I, Gao B, Li Y, Medvedev V, Sun H, Tessler N, Ma Y (2011) Adv Funct Mater 21:3770
Yoon WS, Park SK, Cho I, Oh JA, Kim JH, Park SY (2013) Adv Funct Mater 23:3519
Zhou T, Jia T, Kang B, Li F, Fahlman M, Wang Y (2011) Adv Energy Mater 1:431
Zhou Y, Dai YZ, Zheng YQ, Wang XY, Wang JY, Pei J (2013) Chem Comm 49:5802
Kuo MY, Chen HY, Chao I (2007) Chem Eur J 13:4750
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Cohen AJ, Handy NC (2001) Mol Phys 99:607
Adamo C, Jacquemin D (2013) Chem Soc Rev 42:845
Perrier A, Maurel F, Jacquemin D (2012) Acc Chem Res 45:1173
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09W, revision D.01. Gaussian Inc., Wallingford
Rand BP, Burk DP, Forrest SR (2007) Phys Rev B 75:115327
Li SB, Duan YA, Geng Y, Gao HZ, Qiu YQ, Su ZM (2015) RSC Adv 5:29401
Pan QQ, Li SB, Wu Y, Sun G, Geng Y, Su ZM (2016) RSC Adv 6:81164
Lu T, Chen F (2012) J Comput Chem 33:580
Duan YA, Geng Y, Li HB, Jin JL, Wu Y, Su ZM (2013) J Comput Chem 34:1611
Huang JD, Li WL, Wen SH, Dong B (2015) J Comput Chem 36:695
Delgado MCR, Orduna J, Oliva MM, Casado J, Navarrete JTL (2007) J Chem Phys 127:164704
Garcia G, Granadino-Roldan JM, Hernandez-Laguna A, Garzon A, Fernandez-Gomez M (2013) J Chem Theory Comput 9:2591
Wang GY, Kan YH, Geng Y, Duan YA, Wang L, Wu HQ, Dong XX, Su ZM (2014) Theor Chem Acc 133:1453
Yang S, Kertesz M (2006) J Phys Chem A 110:9771
Liao Y, Yang GC, Feng JK, Shi LL, Yang SY, Yang L, Ren AM (2006) J Phys Chem A 110:13036
Tvingstedt K, Vandewal K, Gadisa A, Zhang F, Manca J, Inganäs O (2009) J Am Chem Soc 131:11819
Staple DB, Oliver PAK, Hill IG (2014) Phys Rev B 89:1719
Scharber MC, Mühlbacher D, Koppe M, Denk P, Waldauf C, Heeger AJ, Brabec C (2006) J Adv Mater 18:789
Nielsen CB, Holliday S, Chen HY, Cryer SJ, McCulloch I (2015) Acc Chem Res 48:2803
Ko S, Mondal R, Risko C, Lee JK, Hong S, McGehee MD, Brédas JL, Bao Z (2010) Macromolecules 43:6685
Murphy AR, Fréchet JM (2007) J Chem Rev 107:1066
Pelzer KM, Darling SB (2016) Mol Syst Des Eng 1:10
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Project No. 21363025) and the Science and Technology Development Project Foundation of Jilin Province (20150101008JC).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Sui, M., Li, S., Pan, Q. et al. Theoretical characterization on photoelectric properties of benzothiadiazole- and fluorene-based small molecule acceptor materials for the organic photovoltaics. J Mol Model 23, 28 (2017). https://doi.org/10.1007/s00894-016-3205-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-3205-8